Transforming medical equipment procurement globally

Understanding Medical Equipment Procurement in India's Rapidly Evolving Healthcare Market

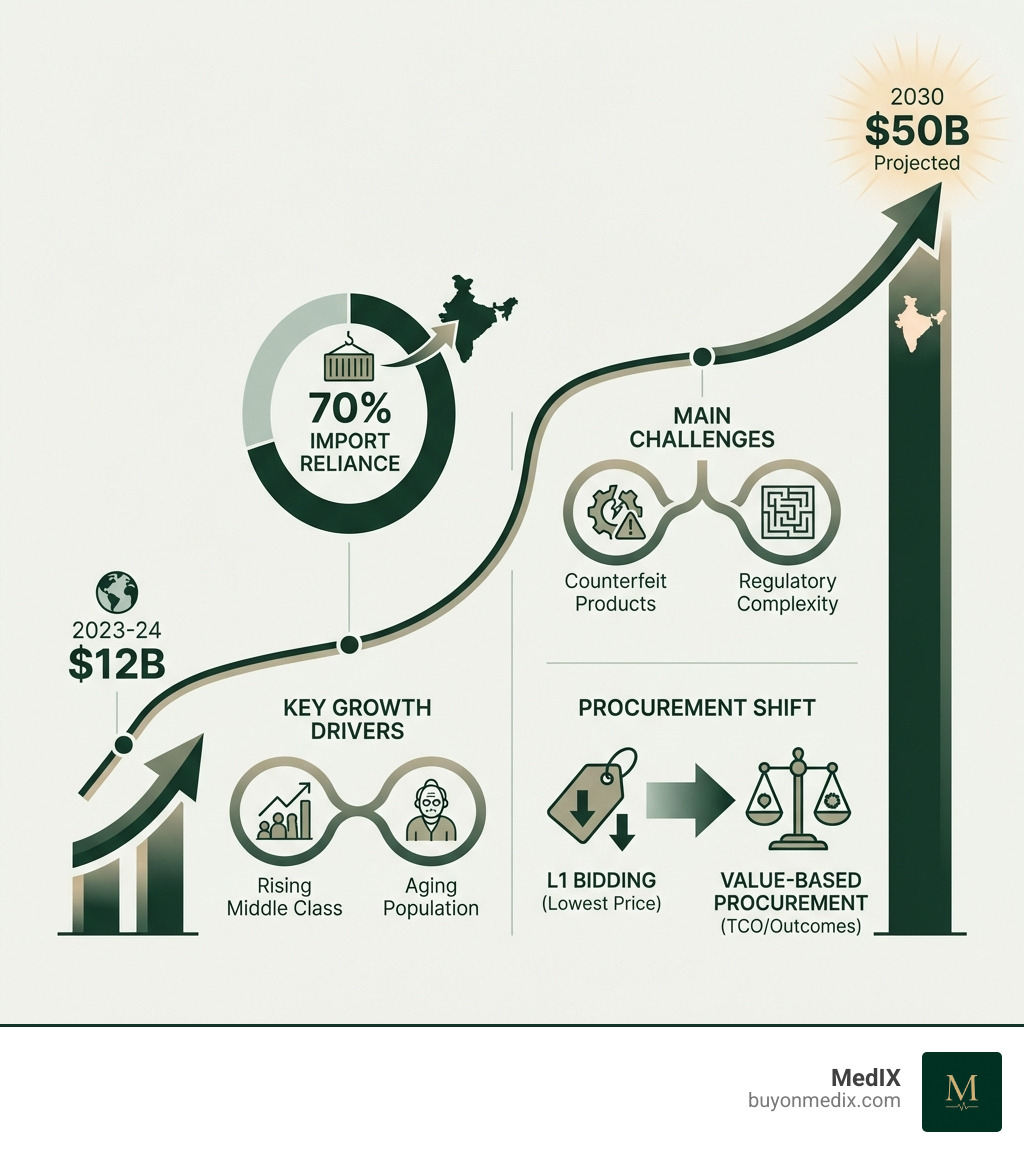
Medical equipment procurement in India is navigating a period of unprecedented change. The market is projected to surge from $12 billion in 2023-24 to $50 billion by 2030, driven by a rising middle class, an aging population, and increased healthcare awareness. Yet despite this growth, India still relies on imports for nearly 70% of its medical devices, creating both opportunities and challenges for procurement managers worldwide.
Key Facts About Medical Equipment Procurement in India:
- Market Size: Growing from $12B (2023-24) to $50B (2030)
- Import Reliance: 70% of medical devices are imported
- Regulatory Body: Central Drugs Standard Control Organization (CDSCO)
- Primary Policy: 'Make in India' initiative favoring domestic manufacturers
- Public Health Spending: Currently under 1.5% of GDP, targeting 2.5% by 2025
- Procurement Methods: Shifting from lowest-bid (L1) to Value-Based Procurement (VBP)
- Key Challenges: Counterfeit products, IP protection, complex import duties, supplier verification
For procurement managers at global healthcare institutions, the Indian market presents a unique landscape. You're dealing with evolving government policies like the Public Procurement Orders that favor local manufacturers, recent relaxations on Global Tender Enquiries for specific devices, and a regulatory environment that's simultaneously opening up and tightening controls.
The traditional lowest-price procurement model is giving way to Total Cost of Ownership (TCO) considerations. As one stakeholder noted in recent research: "Public sector procurement, including the GEM portal currently has bid qualification based on technical specifications and bid finalization based on lowest cost, the value based on outcomes is not seen." This gap between policy and practice creates both friction and opportunity.
Perhaps most critically, it's estimated that between 40% to 70% of medical equipment in low- and middle-income countries is broken, unused, or unfit for purpose. This statistic underscores why proper procurement processes, supplier verification, and lifecycle management aren't just administrative tasks—they're essential to delivering healthcare outcomes.
The Regulatory and Policy Landscape for Medical Equipment Procurement in India
Navigating medical equipment procurement in India requires understanding its regulatory and policy landscape. The Indian government is shaping the market through initiatives that balance domestic manufacturing growth with the need for advanced medical technologies.
The primary regulatory body is the Central Drugs Standard Control Organization (CDSCO), which ensures all medical devices sold or imported into India meet quality, safety, and performance standards. Government policies also significantly impact procurement, especially with ambitious public health spending goals. India allocates under 1.5% of its GDP to public health, with plans to increase this to 2.5% by 2025. This budget increase will impact procurement volumes and strategies nationwide.

'Make in India' and Public Procurement Orders (PPOs)
One of the most influential initiatives is the 'Make in India' program. It boosts domestic manufacturing by encouraging a preference for locally made goods in public procurement. The Public Procurement (Preference to Make in India) Order outlines guidelines for this preference, mandating minimum local content for certain medical devices. This makes it challenging for fully imported products to compete in government tenders.
Production Linked Incentive (PLI) schemes also encourage large-scale manufacturing by offering financial incentives for incremental sales of products made in India. The National Medical Devices Policy 2023 solidifies this commitment, promoting growth through regulatory reform, infrastructure development, and skill improvement. For a deeper dive, explore the government's Public Procurement Policy.
Navigating Tenders and Global Tender Enquiry (GTE) Rules
Public sector medical equipment procurement in India adheres to the General Financial Rules (GFRs), which dictate the tendering process. A key aspect has been the Global Tender Enquiry (GTE), allowing international suppliers to bid. However, the 'Make in India' policy restricted GTEs for tenders up to Rs. 200 crore to give domestic manufacturers a competitive edge.
Recent relaxations have occurred for specific devices. For example, a June 28, 2024, memorandum granted a GTE exemption for 354 medical devices until March 31, 2027. This acknowledges that for some advanced equipment, local manufacturing capacity is insufficient. These relaxations show the government's pragmatic approach: promoting local manufacturing while ensuring access to critical technologies.
For entities procuring Bulk Medical Equipment, staying updated on these dynamic GTE rules is paramount. Central procurement agencies must also exercise due diligence on local manufacturers' claims about local content and capabilities to ensure fairness.
Key Regulatory Problems and Compliance for Importers
Despite its potential, international MedTech companies face challenges in medical equipment procurement in India. A significant concern is intellectual property (IP) protection, as firms worry about safeguarding patented technologies where IP enforcement can be complex.
Substandard and counterfeit products are another pressing issue, threatening brand integrity and patient safety. Ensuring equipment meets stringent quality standards, like those for CE Marked Medical Devices or other Certified Medical Equipment, is critical but complicated by these market realities.
Furthermore, importers face challenges with import duties and health taxes. Price sensitivity in India, with high out-of-pocket expenditure, means cost increases from duties can affect competitiveness. The 'Make in India' initiative's local content requirements also add complexity, as foreign companies must assess how to comply or compete against preferred domestic goods.
Modern Procurement Models: Beyond the Lowest Bid
Historically, public sector medical equipment procurement in India has relied on the L1 (lowest price) bidding model. This approach often overlooked long-term implications, leading to higher overall costs and suboptimal patient outcomes. However, focusing only on the initial price is now seen as a false economy.
The shift is towards a holistic approach that prioritizes value and long-term costs to improve patient outcomes. This means moving beyond a simple Medical Equipment Price Comparison to a comprehensive evaluation. As one expert insight highlights, "The focus on lowest price-based procurement leads to higher total costs due to low quality, breakdowns, and lack of user-friendliness." This underscores the need to consider factors beyond upfront cost.
Embracing Value-Based Procurement (VBP) and Total Cost of Ownership (TCO)
Value-Based Procurement (VBP) is gaining traction as a more sophisticated approach to medical equipment procurement in India. VBP shifts the focus from the lowest price to how a product can deliver desired outcomes, reduce total care costs, and provide long-term benefits. It considers factors like effectiveness, efficiency, and sustainability, not just economy.
Central to VBP is the concept of Total Cost of Ownership (TCO). TCO includes all direct and indirect costs of an asset over its lifecycle, beyond the initial purchase price. Think of it like an iceberg: the purchase price is the tip, while costs like installation, training, consumables, maintenance, and disposal lie hidden beneath the surface.
Our TCO framework includes:
- Purchase Cost: The upfront price of the equipment.
- Operation Costs: Energy consumption, consumables, reagents (for lab equipment), and staff time.
- Maintenance Costs: Planned preventive maintenance (PPM), breakdown repairs, spare parts, and service contracts.
- Training Costs: Initial and ongoing training for users and technicians.
- Disposal Costs: Decommissioning and environmentally responsible disposal at end-of-life.
By comparing the TCO for different options, procurement teams can make more informed decisions that maximize value. This is crucial in India, where research suggests 40% to 70% of medical equipment in low- and middle-income countries is non-functional, often due to a lack of considering these lifecycle costs.

Alternative Procurement Models: Leasing and Rentals
When capital budgets are constrained or technology evolves rapidly, an outright purchase may not be the best solution for medical equipment procurement in India. Alternative models like leasing and rentals offer flexibility and better value.
We often differentiate between:
- Capital Leases: Financing arrangements where the lessee assumes most ownership risks and rewards. The equipment often appears on their balance sheet with an option to purchase at the end of the term.
- Operating Leases: More like rentals, where the lessor retains ownership risks. Payments are expensed, and the equipment is returned or renewed at the end of the term. This is beneficial for equipment with a high obsolescence risk.
Beyond traditional leases, reagent rental agreements are suitable for laboratory equipment. Here, the equipment is provided at little to no upfront cost, and the user pays for consumables. This often includes all-inclusive service, transferring significant risk to the supplier. This model is ideal for rapidly changing technology and allows providers to access advanced diagnostics without a large capital outlay, aligning with the demand for Durable Medical Equipment with comprehensive support.
The Practical Procurement Cycle: From Needs Assessment to Lifecycle Management
Effective medical equipment procurement in India isn't just about making a purchase; it's an end-to-end process that spans from identifying a need to managing the equipment throughout its entire lifecycle. Our goal is to ensure equipment uptime, maximize the value derived from these crucial assets, and ultimately contribute to better patient care. This comprehensive approach is a cornerstone of robust Healthcare Supply Chain Management.
Step-by-Step Guide to medical equipment procurement in India
Let's walk through the typical stages of medical equipment procurement in India:
- Needs Assessment: This is where it all begins. Healthcare facilities must clearly identify what equipment is needed, why it's needed, and what problem it will solve. Is it for a new service, capacity augmentation, or replacing condemned equipment? This stage often involves clinicians, biomedical engineers, and financial teams.
- Documenting Requirements: Once the need is established, precise documentation is critical. This typically involves three sequential documents:
- Procurement Product Profiles: High-level descriptions of the functional needs and desired outcomes.
- Technical Specifications: Detailed functional, performance, safety, and design requirements. These should be generic enough to encourage competition but specific enough to meet clinical needs. The Rajasthan Medical Services Corporation Limited (RMSC), for instance, has detailed guidelines for framing technical specifications, including certifications (USFDA, CE, ISO, BIS) and guarantee periods.
- Procurement Specifications: The actual bidding or tender documents, incorporating the technical specs along with all procurement process details, terms, and conditions.
- Tendering and Bidding: This involves inviting bids from qualified suppliers. In India's public sector, platforms like the Government e-Marketplace (GeM) are often mandatory. The process typically follows a two-bid system: a technical bid (evaluated first for compliance with specifications) and a financial bid (opened only for technically qualified bidders).
- Supplier Evaluation and Selection: This is where the shift from L1 bidding becomes crucial. Beyond price, factors like supplier reputation, service network, warranty, Comprehensive Maintenance Contract (CMC) terms, and adherence to 'Make in India' policies are considered. Our platform, acting as a Hospital Procurement Platform, facilitates rigorous compliance checks and AI-matching to connect buyers with certified suppliers.
- Contracting: Once a supplier is selected, a formal contract is signed, detailing all terms, conditions, delivery schedules, payment terms, and post-sales support.
Health Technology Management (HTM) and Post-Procurement Best Practices
Acquiring equipment is only half the battle; ensuring its effective use and longevity is where Health Technology Management (HTM) comes into play. HTM is a systematic approach to managing medical technology throughout its entire lifecycle, from planning and acquisition to utilization, maintenance, and eventual disposal.
Key post-procurement best practices include:
- Installation and Commissioning: Proper installation, site readiness, and calibration are non-negotiable. Commissioning involves verifying that the equipment functions according to specifications in its operational environment.
- User and Technician Training: Initial and ongoing training for clinical users and biomedical technicians is vital. Poor training is a significant reason why equipment remains unused or is misused.
- Comprehensive Maintenance Contracts (CMC): These are essential for ensuring equipment uptime and managing long-term costs. For major equipment, a 5-year on-site warranty followed by a 5-year CMC is often recommended, as seen in ESIC guidelines. This ensures that maintenance, repairs, and spare parts are covered. The Ministry of Health and Family Welfare has an Approved Report on Modalities for Procurement and Maintenance of Medical Equipment which offers further guidance.
- Equipment Audits: Regular audits assess performance, utilization, and identify equipment that is underused, broken, or nearing condemnation.
- Inventory and Documentation: Maintaining detailed records of each piece of equipment, including its history, service logs, and calibration data, is crucial for effective HTM and for managing your Clinic Medical Supply needs.
Managing Challenges: Counterfeits and Equipment Donations
Two significant challenges in medical equipment procurement in India that require careful management are dealing with counterfeit products and handling equipment donations.
Counterfeit medical products pose a serious risk to patient safety and erode trust in the healthcare system. Our strategy involves rigorously verifying supplier credentials and ensuring that all products meet international quality standards. When looking for Patient Monitor Suppliers or any other critical equipment, always insist on proper certifications and traceable supply chains. This helps mitigate the risk of acquiring substandard or fake devices.
Equipment donations, while well-intentioned, can also present substantial challenges. As our research indicates, between 40% to 70% of medical equipment in low- and middle-income countries is broken, unused, or unfit for purpose, and donated equipment often contributes significantly to this statistic. This can happen because donations may not align with the actual needs of the facility, or there might be a lack of spare parts, trained personnel, or proper infrastructure (like stable power) to operate and maintain them.
To maximize the value of donated equipment and prevent it from becoming a burden, we advocate for adhering to the World Health Organization's (WHO) criteria for sustainable donations:
- Appropriateness: Is the equipment suitable for the setting and the health needs?
- Quality and Safety: Does it meet international standards?
- Affordability and Cost-Effectiveness: Can the recipient afford to operate and maintain it (TCO perspective)?
- Ease of Use and Maintenance: Can local staff operate and service it?
- Conformity with Policies: Does it fit into national health plans and regulations?
For specialized equipment like Microbiology Lab Equipment, these criteria are even more critical, as specific reagents and technical expertise are often required. Procurement managers should have a clear policy for accepting donations, ensuring they truly benefit the healthcare system rather than adding to the pile of non-functional equipment.
Opportunities and Strategies for Success in the Indian Market
The rapid growth of the Indian medical device market presents a compelling landscape for both global and domestic players. With its projected expansion to $50 billion by 2030, understanding where the key opportunities lie and how to strategically approach market entry is crucial for sustainable success in medical equipment procurement in India.
Identifying High-Growth Opportunities in India
Several segments within the Indian medical device market are experiencing significant growth, driven by an increasing burden of chronic diseases, a rising aging population, and improving healthcare infrastructure. These high-potential areas include:
- Diagnostic Imaging: The demand for advanced imaging technologies (MRI, CT scans, ultrasound) is soaring as diagnostic capabilities improve across urban and semi-urban centers.
- Cardiovascular Devices: With a growing prevalence of heart disease, there's a strong market for stents, pacemakers, and other interventional cardiology devices.
- Orthopedic Implants: The increasing number of road accidents and an aging population contribute to a high demand for orthopedic implants and joint replacements.
- In-vitro Diagnostics (IVD): The need for accurate and timely disease diagnosis fuels the growth of IVD reagents, kits, and equipment, including specialized Microbiology Lab Equipment.
- Critical Care Equipment: The expansion of intensive care units (ICUs) and emergency services drives demand for ICU Medical Equipment like ventilators, patient monitors, and anesthesia machines.
- Telemedicine and Digital Health Solutions: The COVID-19 pandemic accelerated the adoption of digital health, creating significant opportunities for remote monitoring devices, telehealth platforms, and health IT solutions.
For a comprehensive overview of the market, including specific high-potential segments and trade data, we recommend consulting U.S. government insights on the India Medical Devices market.
Strategic Approaches for U.S. and Global MedTech Companies
For U.S. and other global MedTech companies looking to thrive in medical equipment procurement in India, a well-thought-out strategy is essential. We've identified several key approaches:
- Thorough Market Research: Before making any moves, invest in in-depth market research. Understand regional healthcare needs, competitive landscapes, pricing sensitivities, and local regulatory specifics. India is not a monolithic market; needs and purchasing power vary significantly across states and types of healthcare facilities.
- Flexible Market Entry Strategies: Consider various entry models beyond direct sales. This could include establishing a local presence, setting up manufacturing facilities (to leverage 'Make in India' incentives), or forming strategic alliances.
- Forming Local Partnerships: Collaborating with experienced local distributors, agents, or even manufacturers is often the most effective way to steer the complex Indian market. Local partners provide invaluable insights into cultural nuances, regulatory processes, and established distribution networks. These Global Medical Device Distributors can be the bridge between your innovative products and the Indian healthcare system.
- Ensuring Regulatory Compliance: This cannot be overstated. Strict adherence to CDSCO regulations, quality standards (e.g., ISO, CE, USFDA), and local content requirements is paramount. Work closely with regulatory experts to ensure all products are properly registered and compliant.
- Focus on Cost-Effectiveness and Value: Given the price sensitivity in India, companies must offer high-quality products that also demonstrate strong value, often through a favorable Total Cost of Ownership. This might involve adapting product features or offering flexible procurement models like leasing.
- Robust Medical Device Logistics: India's vast geography and varied infrastructure demand efficient and reliable logistics for timely delivery, installation, and after-sales service. A strong supply chain is critical for customer satisfaction and equipment uptime.
By adopting these strategies, global MedTech companies can effectively tap into India's burgeoning healthcare market, contributing to its growth while expanding their own global footprint.
Frequently Asked Questions about Medical Equipment Procurement in India
We understand that navigating the complexities of medical equipment procurement in India can raise many questions. Here, we address some of the most common ones we encounter.
What is the 'Make in India' policy's impact on medical device procurement?
The 'Make in India' policy profoundly impacts medical equipment procurement in India by giving preferential treatment to domestically manufactured goods in public procurement tenders. This means that if a local manufacturer can supply a product that meets specified quality and technical standards, they might be favored over an international supplier, even if the international product has a slightly lower price. The policy mandates minimum local content for certain categories of medical devices, which can be a significant hurdle for fully imported products. While it aims to foster local industry growth and reduce import reliance, it can increase competition for foreign companies and sometimes necessitates local manufacturing or assembly for market access, particularly in government contracts.
What is the difference between Total Cost of Ownership (TCO) and the initial purchase price?
The initial purchase price is just one component of the Total Cost of Ownership (TCO). Think of the initial purchase price as the sticker price of a car—it's what you pay upfront. However, owning a car involves much more: fuel, insurance, maintenance, repairs, and eventually, resale value or disposal costs. Similarly, TCO provides a holistic financial estimate by including all direct and indirect costs over the equipment's entire lifecycle. This includes the initial purchase price, but also installation, training, consumables (like reagents for lab equipment), energy consumption, ongoing maintenance, repairs, spare parts, and even the cost of decommissioning and disposal. Focusing solely on the initial purchase price can lead to hidden costs down the line, making a seemingly cheap option far more expensive in the long run.
How can foreign companies steer India's complex regulatory landscape?
For foreign companies, successfully navigating India's complex regulatory landscape for medical equipment procurement in India requires a multi-faceted and proactive approach. Firstly, conducting in-depth market research is crucial to understand specific state-level regulations (where applicable, like the example of Rajasthan's RMSC) and the overall national framework governed by the CDSCO. Secondly, partnering with experienced local distributors or consultants who possess intimate knowledge of the Indian market and regulatory nuances is invaluable. These partners can help with product registration, compliance with quality standards (such as ensuring products are CE Marked Medical Devices or have other necessary international certifications), and understanding local content requirements. Thirdly, companies must ensure all their products have the necessary certifications and registrations from the CDSCO and other relevant bodies. Finally, staying updated on evolving policies, such as changes in GTE rules or 'Make in India' mandates, is critical. This dynamic environment means continuous monitoring and adaptability are key to sustained success.
Conclusion: The Future of Medical Procurement in India
The trajectory of medical equipment procurement in India is undeniably upward, marked by robust growth and continuous policy evolution. We are witnessing a pivotal shift from traditional lowest-bid procurement towards more sophisticated, value-based decision-making. This evolution, driven by the need for better patient outcomes, cost efficiencies, and sustainable healthcare infrastructure, is reshaping how medical devices are acquired and managed across the nation.
The role of technology in streamlining this complex process cannot be overstated. Digital solutions are becoming indispensable, offering greater transparency, efficiency, and connectivity throughout the supply chain. From AI-powered demand forecasting to automated tender management and rigorous compliance checks, technology is ready to revolutionize every aspect of medical procurement. The rise of AI Medical Procurement is a testament to this trend, promising smarter, faster, and more effective purchasing decisions.
In this dynamic environment, digital B2B marketplaces like BuyOnMedix are playing a crucial role. We are simplifying transactions, enhancing transparency, and connecting certified suppliers with healthcare providers globally. Our platform facilitates AI-matching, rigorous compliance checks, and reliable global logistics, ensuring verified equipment and transparent transactions worldwide. To steer this dynamic landscape effectively, leveraging a modern procurement platform that understands global standards and local nuances is key.
Expert voices
Insights from leaders transforming medical equipment procurement






More from our blog
Discover the latest trends in medical technology and procurement
Stay ahead of medical technology
Get the latest insights, research, and market updates delivered straight to your inbox
Ready to transform your procurement
Discover how MedIX can streamline your medical equipment sourcing process


