Transforming medical equipment procurement globally

Why Medical Device Logistics Matters for Patient Care and Business Success
Medical device logistics is the specialized process of managing the storage, handling, transportation, and distribution of medical equipment and devices from manufacturers to healthcare facilities and patients. This complex supply chain ensures life-saving equipment reaches its destination safely, on time, and in full compliance with regulatory standards.
Key components of medical device logistics include:
- Regulatory Compliance - Meeting FDA, ISO, and GMP standards throughout the supply chain
- Temperature Control - Maintaining precise environmental conditions for sensitive devices
- Chain of Custody - Tracking and documenting every movement from factory to patient
- Specialized Handling - Protecting high-value and fragile equipment during transport
- Reverse Logistics - Managing returns, recalls, and equipment refurbishment
As one industry expert noted: "Medical devices so often have the potential to save or change a life." This simple truth underscores why efficient logistics matters so much in healthcare.
The stakes are high and growing. The global medical device logistics market reached USD 15.5 billion in 2022 and is projected to hit USD 27.8 billion by 2030, growing at a 7.5% annual rate. This explosive growth reflects the increasing complexity of medical devices—from simple tongue depressors to advanced surgical robots and wearable health monitors—and the rising demand for personalized medicine.
For procurement managers at global healthcare institutions, these logistics challenges translate into daily operational problems: verifying supplier compliance, ensuring transparent pricing, coordinating international shipments, and maintaining unbroken cold chains. The COVID-19 pandemic exposed just how fragile these supply chains can be, making resilience and visibility more critical than ever.
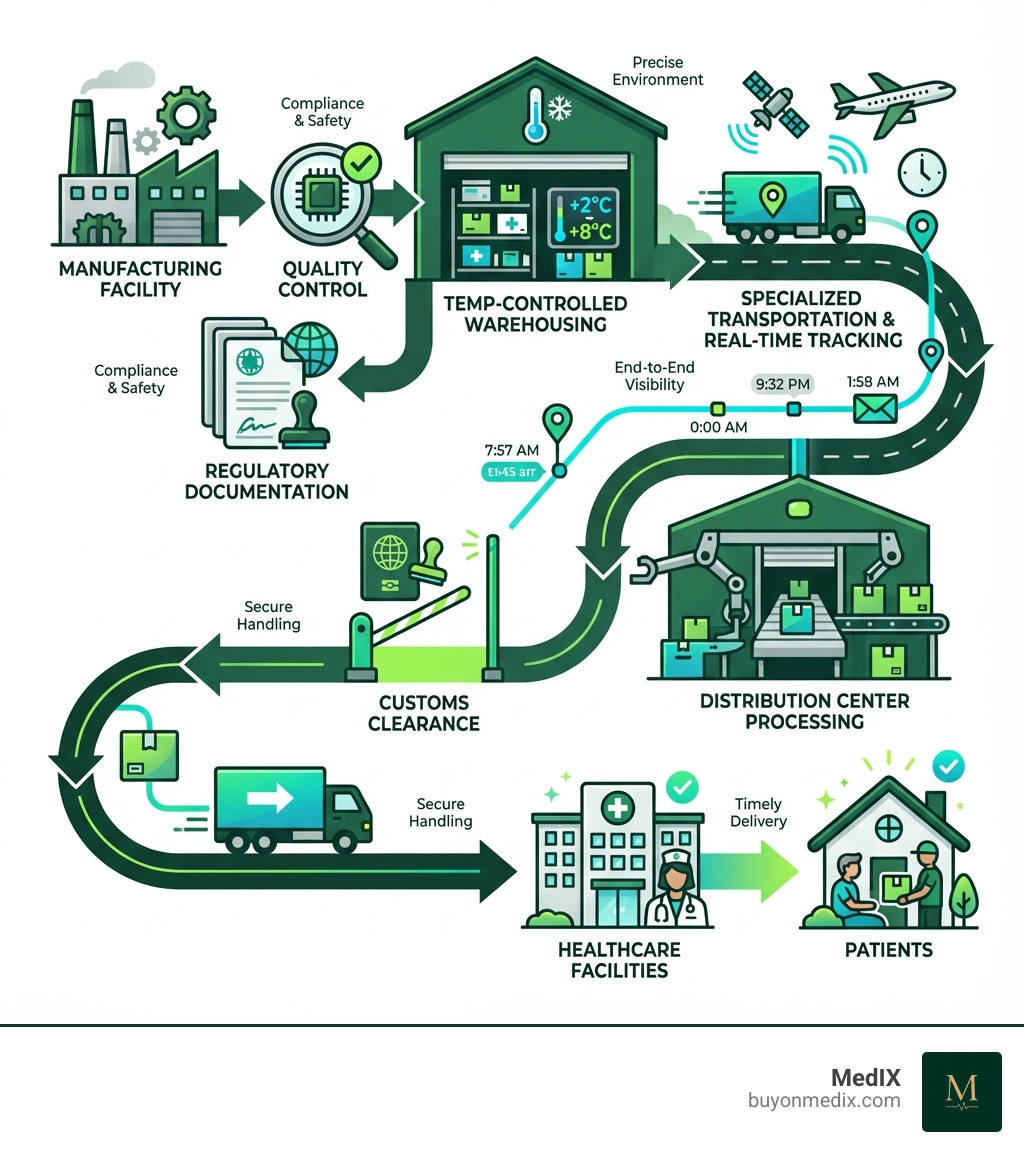
The Core Challenges of Medical Device Logistics
Imagine a surgeon waiting for a critical implant, or a diagnostic machine needing a replacement part. The journey of these items from the factory to the patient's bedside is fraught with unique challenges that set medical device logistics apart from typical supply chains. We're not just moving goods; we're moving life-saving tools, and any hiccup can have profound consequences.
One of the primary challenges is regulatory complexity. Medical devices are among the most regulated products globally, meaning every step of their journey must adhere to strict guidelines. Then there's the issue of temperature sensitivity. Many devices, especially advanced diagnostics or biological implants, require precise environmental controls. A deviation of even a few degrees can render them useless or harmful.
Medical devices often represent high-value equipment, adding another layer of complexity. Advanced surgical robots or MRI machines are vital assets that must be handled with extreme care, demanding robust security and specialized handling throughout the logistics process.
Achieving complete supply chain visibility is another significant hurdle. With multiple stakeholders and international borders, knowing exactly where a device is and its condition can be a daunting task. Yet, this visibility is crucial for proactive problem-solving and ensuring compliance.
Finally, timely delivery is paramount. In healthcare, delays can impact patient outcomes, so punctuality is non-negotiable. Medical devices have precise requirements, are strictly regulated, and demand high levels of availability. Furthermore, healthcare cost pressures mean inventory, obsolescence, and returns must be managed effectively to avoid waste. As experts in the world of medical technology logistics note, these challenges require specialized expertise and infrastructure.
The Evolving Definition of a Medical Device
The definition of a medical device is continuously expanding, significantly impacting medical device logistics. Traditionally, devices ranged from simple tongue depressors to complex surgical instruments like arthroscopy scopes. This core definition still includes items like test kits, oxygen meters, ventilators, syringes, and blood collection tubes.
However, the definition has become much blurrier in recent years, particularly with advancements in technology. As one source aptly puts it, a medical device is "any medical item that is put on you, or in you." This broad scope now includes items like smartwatches, activity trackers, and other forms of wearable technology. These devices, once considered consumer electronics, are increasingly used for health monitoring and diagnosis, thus falling under the medical device umbrella.
This expanding definition presents new logistical challenges. Wearable tech, for instance, may require different storage, packaging, and distribution channels than a sterile surgical implant. It also introduces new regulatory considerations as devices cross the line between consumer and medical goods. Logistics strategies must adapt to this diverse range of products, ensuring every item arrives safely and compliantly.
Navigating the Maze of Regulatory Compliance
In medical device logistics, regulatory compliance is the bedrock of the supply chain. Non-compliance can lead to hefty fines, product recalls, and jeopardized patient safety. Navigating regulations from bodies like the FDA (U.S. Food and Drug Administration), ISO (International Organization for Standardization), and GMP (Good Manufacturing Practices) is therefore a primary challenge.
Adherence to these standards means meticulous attention to detail at every stage. For instance, FDA regulations dictate everything from product labeling and tracking to storage conditions. ISO standards, such as ISO 13485, provide a framework for quality management systems that must be integrated into logistics operations. Good Manufacturing Practices (GMP) ensure products are consistently produced and controlled according to quality standards.
This impacts medical device logistics by demanding:
- Unbroken Chain of Custody: Every movement of a medical device must be recorded, creating a transparent audit trail from the manufacturer to the end-user. This is critical for traceability in case of a recall.
- Comprehensive Documentation: Extensive documentation for all logistics processes, including temperature logs, handling instructions, and proof of delivery, ensures accountability and demonstrates compliance during audits.
- Stringent Quality Control: From the warehouse to the delivery vehicle, every environment must be controlled and monitored to prevent contamination, damage, or degradation of the devices.
- Risk Reduction: Proactively embedding compliance into operations significantly reduces the risk of regulatory violations, product damage, and potential recalls, ultimately safeguarding patient outcomes.
The complexity of these regulations means logistics partners must possess specialized expertise. Meeting these strict requirements is a key differentiator in ensuring the integrity and safety of medical devices worldwide.
Specialized Solutions: How 3PLs and Technology Transform the Supply Chain
The intricate demands of medical device logistics often necessitate specialized solutions that go beyond what a typical in-house logistics department can provide. This is where the power of third-party logistics (3PL) providers and cutting-edge technology truly shines, changing a potentially fragile supply chain into a resilient and efficient network.

Outsourcing logistics to a specialized 3PL allows medical device companies to focus on their core competencies, such as research and development, manufacturing innovations, and patient care. By entrusting the complexities of warehousing, transportation, and distribution to experts, businesses can significantly reduce compliance risks and operational overhead. We believe in leveraging this expertise, recognizing that a specialized partner brings unparalleled knowledge of regulatory requirements, cold chain management, and global distribution networks. This strategic partnership ultimately leads to improved efficiency, reduced costs, and, most importantly, improved patient safety.
The Role of a Specialized Medical Device 3PL
A specialized medical device 3PL provider is more than just a shipping company; it's an outsourced partner that lives and breathes the unique requirements of the healthcare industry. They manage the entire supply chain process, from the moment a device leaves the factory floor to its compliant delivery at a healthcare facility. Their services are tailor-made to handle the delicate nature and stringent regulations surrounding medical equipment.
What exactly do these specialized 3PLs offer?
- Warehousing and Storage: This isn't just any warehouse. Medical device 3PLs provide temperature-controlled storage, often with specific environmental conditions (e.g., humidity control) to protect sensitive devices. Their facilities are designed for cleanliness, security, and strict inventory management.
- Regulatory Compliance Management: They are experts in navigating the labyrinth of FDA, ISO, and GMP standards, ensuring that every step, from documentation to handling, meets global regulatory requirements. This expertise significantly reduces compliance risks and the potential for costly recalls.
- Inventory Management with Serialization and Lot Tracking: Critical for traceability, 3PLs implement advanced systems that allow for precise tracking of each device by serial number and lot, enabling rapid identification and recall if necessary.
- Order Fulfillment and Distribution: This includes specialized handling protocols for delicate or high-value items, custom packaging that meets medical standards, and a robust distribution network capable of reaching healthcare facilities worldwide, often with urgent delivery capabilities.
- Quality Control Packaging: Ensuring devices are protected from damage, contamination, and environmental factors during transit is paramount. 3PLs employ specialized packaging solutions that maintain product integrity.
- Reverse Logistics: They manage the complex process of returns, recalls, refurbishment, and compliant disposal of medical devices, ensuring these activities also adhere to all regulatory standards.
- Scalability: A major benefit is the ability to scale logistics operations up or down based on demand, without the medical device company needing to invest in additional infrastructure or personnel.
By partnering with such a specialized provider, medical device companies can leverage deep expertise, reduce operational burdens, and dedicate more resources to innovation, knowing their products are in safe, compliant hands.
Enhancing Visibility with Technology
In the intricate world of medical device logistics, visibility isn't a luxury; it's a necessity. Knowing where a device is, its condition, and its estimated time of arrival is critical for patient care, inventory management, and regulatory compliance. This is where advanced technology truly shines, changing opaque supply chains into transparent, trackable networks.
One of the most impactful technological advancements is the implementation of track-and-trace capabilities. We use near real-time tracking systems that provide end-to-end product traceability throughout the entire supply chain. This means every component, every package, and every device can be monitored from its origin to its final destination. This isn't just about knowing location; it's about real-time monitoring of environmental conditions, potential impacts, and handling events.
Here's how technology improves visibility:
- Real-time Monitoring: GPS tracking, IoT (Internet of Things) sensors, and advanced telematics provide live updates on shipment location, temperature, humidity, light exposure, and even shock events. This allows us to proactively identify and address potential disruptions before they compromise product integrity.
- Improved Inbound Logistics Visibility: The "first mile" of the supply chain, where raw materials and components are sourced, is often the least visible. By leveraging technology, we can improve inbound logistics efficiency, managing the supply chain for timely and cost-effective material delivery. This ensures that manufacturing processes are not delayed, and critical supplies are always available.
- Just-in-Time (JIT) Systems: For optimized medical supply inventory control, we use JIT systems combined with specialized technology. This minimizes holding costs and reduces the risk of obsolescence by ensuring products arrive exactly when needed, adapting deliveries based on real-time inventory requirements.
- Warehouse Automation: In distribution centers, technology like automated storage and retrieval systems, robotics, and advanced Warehouse Management Systems (WMS) optimize operations. This not only speeds up order management and distribution but also provides precise inventory accuracy and reduces human error.
- Data Analytics: The vast amounts of data collected through these systems are analyzed to identify trends, predict potential bottlenecks, and continuously optimize logistics routes and processes. This continuous improvement cycle is vital for maintaining an agile and resilient supply chain.
By integrating these technological solutions, we achieve comprehensive supply chain visibility, monitoring and tracking all activity from supplier ordering to product delivery. This proactive approach ensures product quality, chain of custody, and ultimately, patient safety.
Mastering Key Components of the Medical Device Supply Chain
Achieving operational excellence in medical device logistics is about more than just moving boxes; it's about safeguarding product integrity and, by extension, patient safety. This demands meticulous end-to-end management of every component within the supply chain, ensuring that each step is executed with precision and compliance.

From the moment a device is manufactured until it reaches the hands of a healthcare professional, we are focused on maintaining its quality and efficacy. This involves a deep understanding of the product's specific requirements, from its ideal temperature range to its fragility. Our commitment to mastering these key components ensures that the life-saving potential of medical devices is never compromised by logistical shortcomings.
Temperature-Controlled Storage and Transportation
Many medical devices, especially advanced diagnostics, certain implants, and biological samples, are extremely sensitive to temperature fluctuations. This makes temperature-controlled storage and transportation, often referred to as cold chain logistics, a non-negotiable aspect of their journey. The cold chain logistics market for medical devices is booming, projected to grow at an 8.2% CAGR from 2023 to 2028, reflecting this critical need.
Critical considerations for temperature control include:
- Refrigerated Transport: For devices requiring temperatures typically between 2-8°C (36-46°F), specialized refrigerated trucks, vans, and air cargo containers are employed. These maintain a consistent cool environment throughout transit.
- Isothermal Containers: These passive containers use insulating materials and phase change materials (PCMs) or gel packs to maintain specific temperature ranges for shorter durations, ideal for last-mile delivery or smaller shipments.
- Cryogenic Storage: For ultra-sensitive biological samples or certain advanced therapies, temperatures can drop below -150°C. This requires specialized cryogenic freezers or liquid nitrogen dewars (containers) that can maintain temperatures as low as -196°C.
- Temperature Monitoring: Throughout the entire cold chain, continuous temperature monitoring is essential. Data loggers and real-time sensors provide alerts if temperatures deviate from the specified range, allowing for immediate intervention. This ensures an unbroken chain of temperature control.
- Validation and Mapping: All temperature-controlled environments, from warehouses to transport vehicles, must be rigorously validated and mapped to ensure uniform temperature distribution and performance under various conditions.
Common temperature ranges for medical devices and related healthcare products include:
- Ambient: 15-25°C (59-77°F) – for devices that are not highly temperature-sensitive but still require protection from extreme heat or cold.
- Refrigerated: 2-8°C (36-46°F) – common for vaccines, certain diagnostic reagents, and some biological samples.
- Frozen: -15 to -25°C (5 to -13°F) – for products like certain blood plasma components or specific diagnostic kits.
- Ultra-low Frozen: -60 to -80°C (-76 to -112°F) – used for specialized biological materials and cell therapies.
- Cryogenic: Below -150°C (e.g., liquid nitrogen at -196°C) – for long-term storage and transport of cells, tissues, and other highly sensitive biologicals.
Our robust cold chain capabilities ensure that even the most delicate medical devices arrive in perfect condition, ready to perform their life-saving function.
Best Practices in Medical Device Logistics Inventory
Effective inventory management is a cornerstone of efficient medical device logistics, especially given the high value, critical nature, and often limited shelf life of these products. It directly impacts healthcare costs, patient access, and operational efficiency. Here are some of our best practices:
- Optimizing Inventory Levels: We strive to strike a delicate balance between having enough stock to meet demand and avoiding overstocking, which ties up capital and increases the risk of obsolescence. This involves sophisticated forecasting models and close collaboration with healthcare providers to understand their needs.
- Obsolescence Management: Medical technology evolves rapidly, meaning devices can become obsolete quickly. We implement proactive strategies to manage obsolescence, such as:
- First-In, First-Out (FIFO) Systems: Ensuring older stock is used before newer stock.
- Clear Expiration Date Tracking: Carefully monitoring and managing products with expiration dates to prevent waste.
- Strategic Discounting/Repurposing: Working with manufacturers to find alternative uses or responsible disposal methods for nearing-obsolete stock.
- Returns Management: Handling returns of medical devices is complex due to regulatory and quality control requirements. Our processes ensure that returned items are inspected, categorized (e.g., for repair, refurbishment, or disposal), and handled in compliance with all relevant standards.
- Just-in-Time (JIT) Inventory: For many medical supplies, JIT systems are employed to minimize inventory holding costs and reduce waste. By leveraging technology and strong supply chain visibility, we ensure that products arrive exactly when needed, optimizing the flow of goods and reducing the need for extensive on-site storage.
- Serialization and Lot Tracking: This is a critical practice for ensuring traceability and enabling rapid recalls if necessary. Each device is assigned a unique serial number, and its journey is tracked by lot, allowing for precise identification and management throughout the supply chain. This is not just a best practice; it's often a regulatory requirement.
By implementing these best practices, we help medical device companies and healthcare facilities manage their inventory efficiently, reduce costs, and maintain the highest standards of product quality and safety.
The Critical Role of Reverse Logistics
In medical device logistics, the journey doesn't always end with delivery. Sometimes, products need to move back through the supply chain, a process known as reverse logistics. This is a critical, complex, and highly regulated aspect that demands as much precision as forward logistics, especially when dealing with patient safety.
Reverse logistics encompasses several key scenarios:
- Product Returns: Devices might be returned due to damage, incorrect orders, or end-of-lease agreements. Our process ensures these returns are handled efficiently, inspected, and routed appropriately, whether for restocking, repair, or disposal.
- Recalls: In the event of a product defect or safety concern, a rapid and compliant recall process is paramount. Reverse logistics facilitates the swift retrieval of affected devices from healthcare facilities and patients, minimizing risk and adhering to strict regulatory guidelines.
- Refurbishment and Repair: Many high-value medical devices can be refurbished or repaired, extending their lifespan and reducing costs. Reverse logistics manages the collection, transport to specialized repair centers, and reintroduction of these devices back into the supply chain.
- Compliant Disposal: Medical devices, particularly those that are contaminated, expired, or deemed unsafe, require compliant disposal. This often involves specialized waste management protocols, including handling sharps and hazardous materials, to protect public health and the environment. We ensure that all disposal methods adhere to local and international regulations.
- Surgical Kit Replenishment: For reusable surgical kits, reverse logistics involves collecting used kits from hospitals, transporting them to sterilization and replenishment centers, and then preparing them for their next use. This cyclical process is vital for the efficiency of surgical operations.
The efficiency and compliance of reverse logistics are crucial. As one of our sources highlighted, specialized 3PLs manage reverse logistics for returns, recalls, and refurbishment. Even unattended delivery options, managed by professional drivers, can improve security and efficiency in stocking and reverse logistics, ensuring a seamless flow even in the backward direction. Our expertise in this area helps mitigate risks, optimize resource utilization, and maintain regulatory integrity throughout the entire lifecycle of a medical device.
Frequently Asked Questions about Medical Device Logistics
What are the primary benefits of partnering with a medical device 3PL?
Partnering with a specialized 3PL provides a wealth of benefits custom to the unique demands of medical device logistics. Firstly, it offers unparalleled expertise in regulatory compliance, ensuring that all storage, handling, and transportation processes meet stringent FDA, ISO, and GMP standards. This significantly reduces compliance risks and the potential for costly recalls. Secondly, it allows medical device companies to focus on core activities like research and development, innovation, and manufacturing, rather than diverting resources to complex logistics operations. Thirdly, 3PLs offer scalable solutions for warehousing, temperature-controlled storage, and global distribution, enabling businesses to adapt quickly to changing market demands without heavy capital investment. Additionally, they provide improved supply chain visibility through advanced technology, improving efficiency and accountability.
How is supply chain visibility achieved for medical devices?
Supply chain visibility for medical devices is achieved through a multi-faceted approach leveraging advanced technology. This primarily includes real-time track-and-trace systems that monitor every movement of a device from its point of origin to its final destination. GPS monitoring provides precise location data, while IoT sensors integrated into packaging or containers continuously monitor critical environmental conditions such as temperature, humidity, and shock. Integrated Warehouse Management Systems (WMS) and Transportation Management Systems (TMS) provide a comprehensive digital overview of inventory levels, order status, and shipment progress. This technological ecosystem allows for proactive identification of potential issues, ensures product integrity, and provides a complete, auditable view of the device's journey, from the first mile of inbound logistics to the last-mile delivery.
What types of transport containers are used for medical devices?
A wide array of specialized transport containers is essential in medical device logistics to ensure product integrity and safety. These include:
- Insulated Boxes: Often used for shorter transit times or last-mile delivery, these containers use insulating materials and gel packs or phase change materials to maintain specific temperature ranges (e.g., refrigerated or frozen) for several hours.
- Active Refrigerated Containers: These are sophisticated units, often self-powered, that can actively cool or heat their contents to maintain precise temperature ranges (e.g., 2-8°C, -20°C) over long distances and for extended periods, suitable for air, sea, or road transport.
- Cryogenic Containers (Dewars): Made from materials like aluminum with multilayer insulation, these are designed to hold liquid nitrogen, maintaining ultra-low temperatures (e.g., -196°C) for highly sensitive biological samples or therapies requiring cryopreservation.
- Shielded Containers: Made from materials like tungsten or lead, these are specifically designed for the safe transport of radioactive materials, ensuring protection for handlers and the environment.
- Durable, Sealed Totes and Cases: These robust containers, often made of polyethylene or polypropylene, are used for sterile surgical kits, high-value equipment, or medical waste. They are designed for security, impact protection, and sometimes hermetic sealing to prevent contamination.
- Waste Containers: Specialized containers, often UN-certified, are used for the safe collection and transport of sharps, biohazardous waste, and other medical waste materials, ensuring compliant disposal.
The choice of container depends on the device's specific requirements, including temperature sensitivity, fragility, hazardous properties, and required transit duration.
Conclusion: Ensuring a Resilient and Reliable Medical Device Supply Chain
The journey of a medical device from factory to patient is a testament to the intricate dance of logistics, technology, and human expertise. We've explored the significant challenges inherent in medical device logistics, from the changing definition of a medical device and the stringent regulatory landscape to the critical demands of temperature control and the complexities of reverse logistics. The sheer value and life-saving potential of these products mean that every logistical decision carries immense weight.
However, we've also seen how specialized solutions, particularly through strategic partnerships with medical device 3PL providers and the integration of cutting-edge technology, are changing these challenges into opportunities for operational excellence. From real-time track-and-trace capabilities and intelligent inventory management to robust cold chain solutions and compliant reverse logistics, technology and expertise combine to create resilient and reliable supply chains.
The goal of medical device logistics is singular and profound: to ensure that critical medical equipment reaches those who need it, exactly when they need it, in perfect condition. This commitment to patient safety and well-being drives every aspect of our operations.
At MedIX, we understand these complexities intimately. As a global B2B marketplace, we are dedicated to simplifying procurement for hospitals and clinics worldwide by connecting them with certified medical equipment suppliers. Our platform leverages AI-matching, rigorous compliance checks, and reliable global logistics to ensure verified equipment and transparent transactions, making the critical journey of medical devices as smooth and secure as possible.
We believe that by embracing innovation and collaboration, we can continue to build a future where medical device logistics is a seamless, invisible force for good, underpinning healthcare systems globally.
To explore a vast range of certified medical equipment and experience streamlined procurement, we invite you to visit our marketplace: Explore a wide range of certified medical equipment.
Expert voices
Insights from leaders transforming medical equipment procurement






More from our blog
Discover the latest trends in medical technology and procurement
Stay ahead of medical technology
Get the latest insights, research, and market updates delivered straight to your inbox
Ready to transform your procurement
Discover how MedIX can streamline your medical equipment sourcing process



