Transforming medical equipment procurement globally
Why CE Marked Medical Devices Matter for Your Procurement
CE marked medical devices are products that have passed European conformity assessment, allowing them to be sold across the European Economic Area (EEA). For those sourcing medical equipment, understanding the CE mark is essential for ensuring compliance, safety, and avoiding costly procurement mistakes.
Key Facts About CE Marked Medical Devices:
- Meaning: The CE mark (Conformité Européenne) is the manufacturer's declaration that a device meets all applicable EU safety and performance requirements.
- Scope: It is mandatory for medical devices sold in all 33 EEA member countries.
- Responsibility: The manufacturer holds primary responsibility, though importers and distributors also have legal obligations.
- Legal Basis: The process is governed by the Medical Devices Regulation (EU) 2017/745 (MDR).
- Benefit: It enables the free movement and sale of compliant devices throughout the European market.
The CE mark represents a rigorous process ensuring devices meet strict safety standards. For procurement managers, verifying CE marking is a critical first step in supplier vetting. Devices without a valid CE mark cannot be legally sold in Europe and could expose your institution to significant compliance and liability risks.
The regulatory landscape has become stricter under the MDR. Devices CE marked under this regulation must demonstrate stronger clinical evidence, maintain improved post-market surveillance, and provide greater transparency through systems like the EUDAMED database and Unique Device Identification (UDI) codes. Understanding CE marking helps you verify regulatory compliance and procure safe, legal medical equipment.
What is CE Marking and Why is it Essential?
At its core, the CE mark ('Conformité Européenne') is a mandatory declaration by a manufacturer that a product complies with all applicable European health, safety, and performance requirements. For CE marked medical devices, this means the product has undergone a rigorous assessment to ensure it is safe and performs as intended.
The CE mark is a passport for market access, required for distributing and selling medical devices across the 33 countries of the European Economic Area (EEA). Once a device bears the CE mark, it can be marketed freely within this zone, ensuring a common standard of quality and safety. The primary purpose is to guarantee that products meet high health and safety standards, protecting both patients and healthcare professionals. This legal conformity is non-negotiable for accessing the European market.
A Guide to the application of the EU Regulation can be found here: A Guide to the application of the EU Regulation can be found here
The Legal Foundation for Medical Devices
The regulatory framework for medical devices in Europe is built on the Medical Devices Regulation (MDR), Regulation (EU) 2017/745. This comprehensive regulation, which took full effect on May 26, 2021, reshaped the requirements for CE marked medical devices. It replaced the previous Medical Device Directive (MDD) and Active Implantable Medical Device Directive (AIMD). For in vitro diagnostic products, the In Vitro Diagnostic Medical Devices Regulation (IVDR), Regulation (EU) 2017/746, came into force on May 26, 2022.
These new regulations significantly lift the bar for conformity assessment, clinical evidence, and post-market surveillance. Manufacturers often use harmonised standards, such as EN ISO 14971 for risk management, to demonstrate conformity with the General Safety and Performance Requirements (GSPRs) outlined in the regulations.
Who is Responsible for Compliance?
Responsibility for CE marking compliance is shared among several "economic operators" in the supply chain.
Manufacturer: Holds the ultimate responsibility for ensuring the device meets all GSPRs, preparing technical documentation, conducting clinical evaluations, implementing post-market surveillance, and engaging a Notified Body when required.
Authorised Representative (EC REP): Mandatory for manufacturers based outside the EU, this entity acts on the manufacturer's behalf within the EU, serving as the point of contact for authorities and ensuring regulatory obligations are met.
Importer: Before placing a device on the market, importers must verify it has the CE marking, an EU Declaration of Conformity, and proper labeling. They must also ensure the device is registered in EUDAMED and that the manufacturer has fulfilled UDI obligations.
Distributor: Must verify that the device bears the CE marking and is accompanied by the required documentation and information from the manufacturer. They must also ensure proper storage and transport conditions.
This network of accountability ensures that only compliant and safe CE marked medical devices reach the European market.
The Path to Compliance for CE Marked Medical Devices
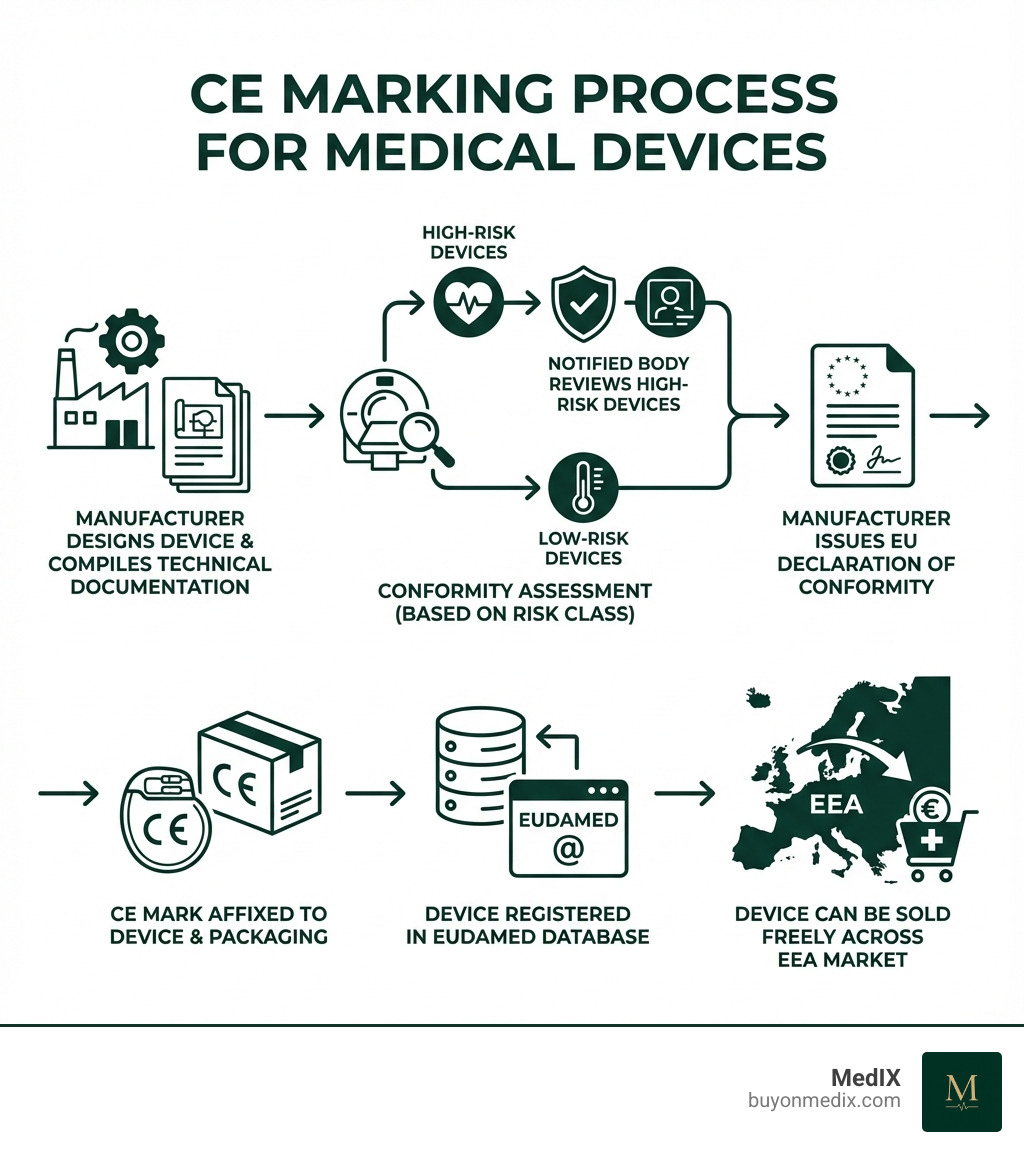
Obtaining the CE mark for a medical device is a systematic, risk-based process. The journey begins with classifying the device into one of four classes—Class I, Class IIa, Class IIb, or Class III—based on its intended purpose, invasiveness, and overall risk. Class III represents the highest risk.
The higher the risk class, the more stringent the conformity assessment. For most devices above Class I, the involvement of a Notified Body is mandatory. These are independent organizations designated by EU Member States to assess whether a device meets the required criteria. Central to the process is the technical documentation, which serves as proof of compliance. Once conformity is demonstrated, the manufacturer issues an EU Declaration of Conformity and affixes the CE mark.
Step-by-Step Conformity Assessment Process
Manufacturers can systematically achieve compliance for their CE marked medical devices by following these steps:
1. Classify your device: Determine the device's risk class (I, IIa, IIb, or III) based on its intended use and characteristics. This dictates the required conformity assessment route.
2. Implement a Quality Management System (QMS): A robust QMS, typically compliant with ISO 13485:2016, is mandatory for most devices to ensure consistent quality and regulatory compliance.
3. Compile the Technical File: This comprehensive dossier provides evidence of conformity, including design specifications, risk analysis, clinical evaluation data, and labeling. It must be kept for at least 10 years after the last device is placed on the market.
4. Appoint an EU Authorised Representative: If located outside the EU, you must appoint an Authorised Representative (EC REP) to act as your legal liaison within the European Union.
5. Engage a Notified Body: For Class IIa, IIb, and III devices, a Notified Body must audit your QMS and review your Technical File. A positive assessment results in a CE certificate.
6. Issue a Declaration of Conformity and affix the CE mark: After completing all steps, the manufacturer signs an EU Declaration of Conformity. The CE mark can then be affixed to the device, its packaging, and instructions, along with the Notified Body's four-digit ID number if applicable.
Essential Documentation for CE Marked Medical Devices
Comprehensive documentation is the foundation of CE marking, serving as verifiable proof of compliance. Key documents include:
- Technical File: A complete dossier on the device's design, manufacturing, intended purpose, risk analysis, and verification/validation results.
- Clinical Evaluation Report (CER): A systematic analysis of clinical data that verifies the device's safety and performance. The MDR has significantly stricter requirements for clinical evidence.
- Risk Management File: Developed according to standards like ISO 14971, this file identifies, evaluates, and controls risks throughout the device's lifecycle.
- Post-Market Surveillance (PMS) Plan: Outlines the manufacturer's process for continuously monitoring the device's safety and performance after it is on the market.
- Labelling and Instructions for Use (IFU): Must be clear, accurate, and provide all necessary information for safe use, including warnings and storage conditions.
The New Era: How the EU MDR Reshapes CE Marking
The Medical Devices Regulation (EU) 2017/745 (MDR) introduced a new era for CE marked medical devices, with a stronger focus on patient safety and transparency. The MDR aims to create a more robust, predictable, and sustainable regulatory framework across the EU.
A key change is the push for greater transparency, facilitated by the EUDAMED database. Once fully operational, this centralized European database will make information on CE marked medical devices publicly accessible, improving oversight for authorities and users. Another pivotal innovation is the Unique Device Identification (UDI) system. All medical devices must have a UDI, which improves traceability, strengthens safety monitoring, and helps combat counterfeiting.

The Impact of MDR on CE Marked Medical Devices
The MDR has profoundly impacted every stage of a device's lifecycle. Key changes include:
- Stricter Clinical Evidence: Manufacturers must provide more robust clinical evidence to demonstrate safety and performance. For some high-risk devices, expert panels review clinical evaluations before certification.
- Improved Post-Market Surveillance: Post-Market Clinical Follow-up (PMCF) is now a continuous process to monitor a device's safety and performance throughout its lifetime, helping to identify unforeseen risks.
- Person Responsible for Regulatory Compliance (PRRC): Manufacturers must appoint a PRRC with expertise in medical device regulations to oversee compliance activities.
- Liability and Financial Coverage: Manufacturers must have sufficient financial coverage to compensate patients for any harm caused by a defective device.
The full text of the Medical Devices Regulation can be found here
Transitioning from MDD to MDR
The transition from the old Medical Device Directive (MDD) to the MDR has been a complex, multi-year process. Devices with valid CE certificates under the MDD, known as legacy devices, can remain on the market for a limited time under specific conditions.
The transition has been challenged by limited Notified Body capacity under the new, stricter MDR requirements. To address this, the EU has introduced extended deadlines for legacy devices to become MDR-compliant. The deadlines are 2027 for high-risk devices (Class III and IIb implantables) and 2028 for medium and lower-risk devices. During this period, both MDR-compliant and legacy MDD-compliant devices will legally co-exist on the market.
CE Marking vs. Other Global Standards
While the CE mark is essential for the European market, it is not a universal certification. Different countries have their own regulatory systems. This means a CE marked medical device cannot automatically be sold in other major markets, like the US, without undergoing their respective approval processes.
| Feature | CE Marking (EU/EEA) | FDA Approval (USA) | UKCA Marking (Great Britain) |
|---|---|---|---|
| Legal Basis | MDR (EU 2017/745), IVDR (EU 2017/746) | FD&C Act, various regulations (e.g., 21 CFR Part 820) | UK MDR 2002 (as amended), future UKCA regulations |
| Scope | 33 EEA member countries | United States | England, Scotland, Wales (Northern Ireland follows EU rules) |
| Symbol | CE Mark | No single FDA "mark" for devices; "FDA Cleared" or "FDA Approved" | UKCA Mark |
| Classification | Class I, IIa, IIb, III (MD); A, B, C, D (IVD) | Class I, II, III | Class I, IIa, IIb, III (MD); A, B, C, D (IVD) (mirrors EU) |
| Conformity Assessment | Manufacturer Declaration (Class I), Notified Body involvement (Higher Classes) | 510(k) Pre-Market Notification, PMA (Pre-Market Approval) | Manufacturer Declaration (Class I), UK Approved Body involvement (Higher Classes) |
| QMS Standard | ISO 13485:2016 (harmonised) | 21 CFR Part 820 (Quality System Regulation) | ISO 13485:2016 (recognised) |
| Database | EUDAMED (in progress) | GUDID (Global UDI Database), Device Registration | MHRA database (for registration) |
| Post-Market | PMS, PMCF, Vigilance, UDI | Adverse Event Reporting (MDR), Recalls, UDI | PMS, Vigilance (mirrors EU) |
CE Marking vs. FDA Approval
The European CE marking system and the U.S. Food and Drug Administration (FDA) process are the two most influential regulatory systems globally. The primary difference is geographical: CE marking is for the EEA, while FDA approval/clearance is for the United States. Their regulatory philosophies also differ. The FDA is often seen as more pre-market focused, with its Pre-Market Approval (PMA) and 510(k) clearance pathways. In contrast, the EU's MDR, while strengthening pre-market requirements, places a heavy emphasis on continuous Post-Market Surveillance (PMS) and lifecycle management.
CE Marking vs. UKCA and Other Marks
Following Brexit, the UKCA mark (UK Conformity Assessed) is required for medical devices sold in Great Britain (England, Scotland, and Wales). Northern Ireland continues to follow EU rules and use the CE mark. Currently, CE marked medical devices can still be placed on the Great British market until June 30, 2028, but the UKCA mark will eventually become mandatory. The UK's technical requirements largely mirror the EU's MDR, but assessment must be done by a UK Approved Body.
Other marks, like the German GS Mark (Geprüfte Sicherheit), are voluntary safety certifications. Unlike the mandatory CE mark for market access, these are optional symbols of improved safety testing.
Benefits and Consequences of CE Marking
The CE mark is more than a compliance hurdle; it is a gateway to opportunity and a foundation of trust. For manufacturers, patients, and procurement professionals, the benefits of CE marked medical devices are profound.
The most significant advantage is market expansion. The CE mark allows manufacturers to sell products in all 33 EEA countries, a unified market of over 450 million people. Beyond market access, the CE mark fosters immense consumer and professional confidence, serving as a clear statement that a device meets stringent European health and safety standards. This assurance is critical for patients and healthcare professionals who rely on the safety and reliability of their equipment.
The Advantages for Manufacturers and Patients
For manufacturers, CE marking offers clear advantages:
- EEA Market Access: Opens a massive economic bloc for sales and distribution.
- Improved Credibility: The CE mark is a globally recognized symbol of quality and safety, boosting a manufacturer's reputation.
- Better Safety Data: The MDR's rigorous requirements for clinical evidence and post-market surveillance provide invaluable data for continuous product improvement.
Patients are the ultimate beneficiaries:
- Improved Safety: Devices marked under the MDR comply with stricter safety requirements and undergo more rigorous controls.
- Increased Transparency: The EUDAMED database and more detailed labeling provide greater visibility into device specifications, clinical data, and safety information, empowering patients and healthcare professionals.
The Risks of Non-Compliance
The consequences of incorrect or non-compliant CE marking are severe. Regulatory bodies in the EU can impose devastating repercussions.
- Market Prohibition and Withdrawal: Non-compliant devices cannot be legally sold in the EEA. Authorities can mandate product recalls, a costly and logistically complex process.
- Financial Penalties: Administrative fines for improper CE labeling can be substantial, reaching up to €1 million in some member states.
- Legal Action: Manufacturers face the risk of legal action from authorities, competitors, or patients harmed by a non-compliant device.
- Reputational Damage: A recall or non-compliance finding can irrevocably harm a brand's image, eroding the trust of healthcare professionals and patients. For procurement managers, sourcing non-compliant devices exposes their institutions to similar risks.
Conclusion
The CE mark for medical devices is a critical symbol of trust, safety, and market access within the European Economic Area. It signifies a manufacturer's commitment to meeting Europe's stringent health and safety standards, providing essential assurance for patients and healthcare providers.
The MDR has improved patient safety through stricter clinical evidence requirements, improved traceability with the UDI system, and fostered greater transparency via the EUDAMED database. While navigating the regulatory landscape can be complex, the benefits of compliance—including market expansion and consumer confidence—are undeniable. Conversely, the risks of non-compliance, from market withdrawal to severe reputational damage, highlight the necessity of adhering to these regulations.
At MedIX, we understand the critical importance of sourcing verified, CE marked medical devices. Our global B2B marketplace simplifies procurement by connecting certified medical equipment suppliers with hospitals and clinics worldwide. We integrate rigorous compliance checks and reliable global logistics to ensure you receive verified equipment and enjoy transparent transactions. Trust MedIX to help you secure safe and compliant medical devices for your institution.
Find certified medical equipment on our marketplace: Find certified medical equipment on our marketplace
Expert voices
Insights from leaders transforming medical equipment procurement






More from our blog
Discover the latest trends in medical technology and procurement
Stay ahead of medical technology
Get the latest insights, research, and market updates delivered straight to your inbox
Ready to transform your procurement
Discover how MedIX can streamline your medical equipment sourcing process



