Transforming medical equipment procurement globally
Why Certified Medical Equipment Matters for Healthcare Procurement
Certified medical equipment refers to medical devices and technologies that have been verified to meet specific quality, safety, and performance standards through recognized certification processes like ISO 13485 and MET Mark. For procurement managers, understanding these certifications is essential for sourcing equipment that protects patients, ensures regulatory compliance, and delivers long-term operational value.
Quick Answer: What You Need to Know About Certified Medical Equipment
- Key Certifications: ISO 13485 (quality management for medical devices) and MET Mark (safety and performance standards)
- Equipment Types: New, refurbished, recertified, and pre-owned devices
- Cost Savings: Recertified equipment typically costs 30-70% less than new without compromising safety
- Documentation: Look for calibration reports, safety testing, and warranty coverage
- Avoid: NTEP certification (designed for commercial scales, not medical devices)
The cost of medical equipment has become a major challenge for healthcare facilities worldwide. With new devices becoming increasingly expensive, procurement managers face intense pressure to balance budget constraints against the need for reliable, safe equipment. At the same time, navigating the complex landscape of certifications, supplier credibility, and international logistics can feel overwhelming.
This guide cuts through the confusion. We'll explain which certifications actually matter for medical devices, why ISO 13485 and MET certification should be your top priorities, and how recertified equipment offers a smart alternative to purchasing new. You'll also learn what to look for when vetting suppliers, what documentation should accompany certified equipment, and how to ensure your purchases meet both regulatory requirements and your facility's operational needs.
Whether you're sourcing diagnostic imaging systems, patient monitors, or EMS equipment, understanding certification standards is the foundation of confident procurement decisions.
Decoding Key Certifications: What Every Healthcare Facility Needs to Know
When it comes to procuring medical equipment, certifications are not just fancy badges; they are critical safeguards. They assure us that the equipment we bring into our facilities is built to a certain standard, protecting both our patients and our staff. These certifications are vital for quality assurance, effective risk management, and ensuring global standards are met, ultimately contributing to the cost-effectiveness and operational efficiency of a healthcare facility. We need to look beyond the price tag and understand what these certifications truly represent. ISO, for example, develops standards that ensure the safety, performance, and reliability of medical equipment and devices worldwide. You can learn more about ISO's work in this sector here: ISO - Medical equipment.
The Gold Standards: ISO 13485 and MET Mark
If there are two certifications that should be at the top of your checklist for certified medical equipment, they are ISO 13485 and MET Mark. Think of them as the gold standard for quality and safety.
ISO 13485 is a globally recognized standard for quality management systems specifically focused on the medical device industry. It outlines comprehensive requirements for a quality management system that ensures manufacturers consistently design, develop, produce, install, and service medical devices that are safe and effective. It's not about certifying the product itself, but rather the system used to create it. This means that a manufacturer holding ISO 13485 certification has robust processes in place to minimize risks, ensure product quality, and comply with regulatory requirements. For us, this translates to confidence in the manufacturing process and the integrity of the equipment. For a deeper dive into this crucial standard, visit: More about ISO 13485.
MET certification, on the other hand, signifies that the equipment meets international standards for safety and performance. This certification often involves rigorous testing to ensure the device operates as intended without posing undue risks to patients or users. It covers aspects like electrical safety, electromagnetic compatibility, and mechanical integrity, all crucial for the complex environment of a healthcare facility. Together, ISO 13485 and MET certification provide a powerful assurance: the equipment is made well, and it works safely.
The Benefits of Choosing ISO 13485 and MET Certified Medical Equipment
Choosing certified medical equipment that adheres to ISO 13485 and MET standards brings a host of benefits that directly impact patient care and operational efficiency:
- Improved Patient Safety: At the core of these certifications is patient well-being. By adhering to strict quality management and safety standards, the likelihood of equipment malfunction or harm is drastically reduced. We can rest easier knowing our patients are in safe hands, supported by reliable technology.
- Reduced Device Failure and Downtime: Equipment that is manufactured under ISO 13485 and tested for MET compliance is inherently more reliable. This means fewer unexpected breakdowns, less maintenance, and ultimately, less downtime for critical procedures. This positively impacts our ability to provide continuous care.
- Improved Operational Efficiency: When equipment works as expected, our staff can perform their duties without interruption or concern. This seamless operation contributes significantly to overall efficiency, allowing us to serve more patients and optimize resource allocation.
- Ensured Performance and Accuracy: MET certification specifically attests to the performance and accuracy of a device. For diagnostic tools or treatment delivery systems, this is non-negotiable. We need to trust that the readings are correct and the treatments are precise.
- Global Accessibility and Compliance: These international standards facilitate global trade and acceptance of medical devices. For a global marketplace like ours, this means easier access to high-quality equipment from reputable manufacturers worldwide, streamlining procurement and ensuring compliance across different regulatory landscapes.
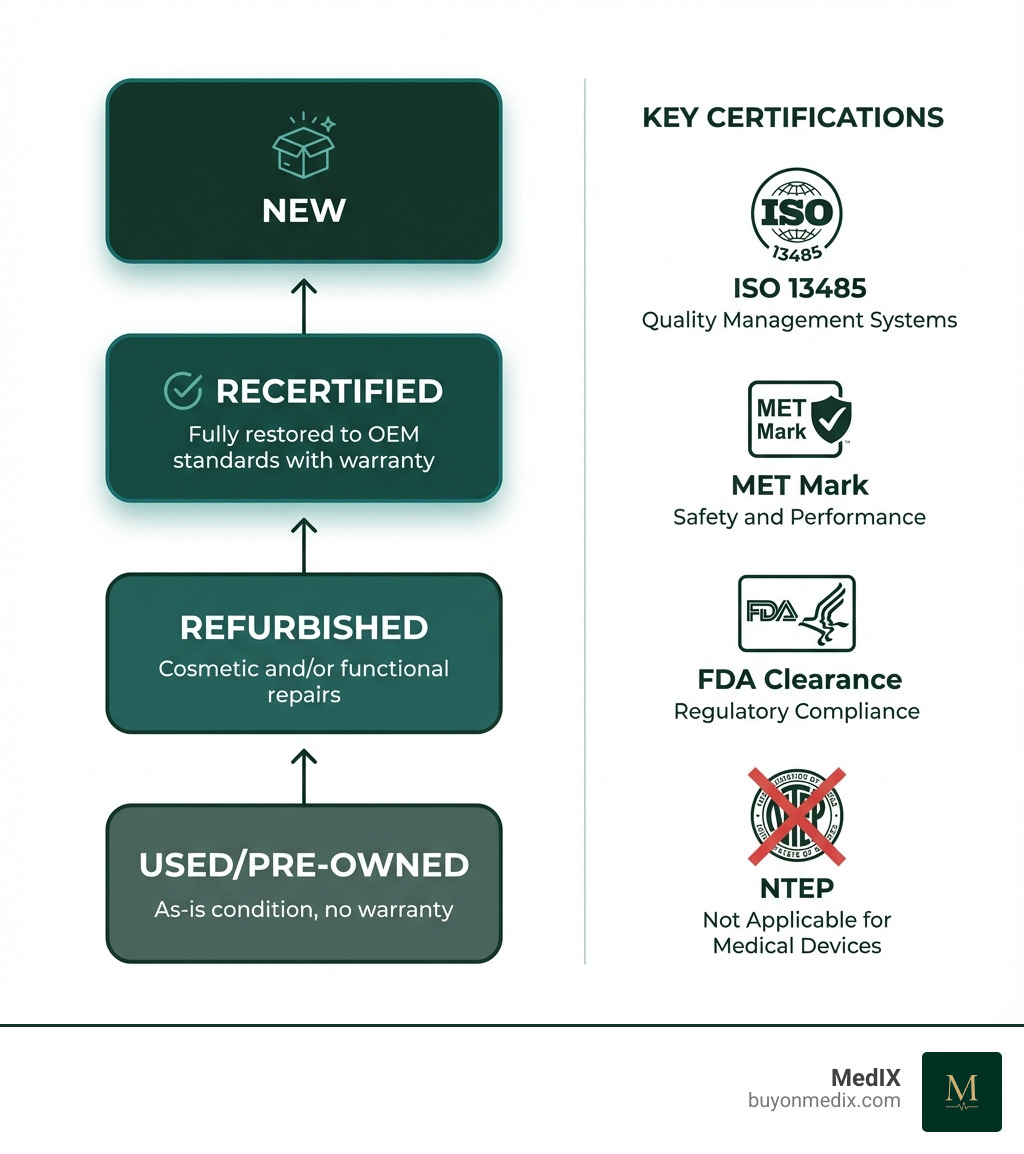
Understanding NTEP Certification and Its Place (or Lack Thereof)
Now, let's talk about a certification that often causes confusion in the medical equipment world: NTEP. While not inherently bad, NTEP (National Type Evaluation Program) certification is generally not recommended for medical equipment, and here's why.
NTEP certification primarily pertains to weighing and measuring devices used in commercial trade, like the scales you might find at a grocery store or a shipping facility. Its focus is on ensuring accuracy and consistency for commercial transactions. While accuracy is certainly important for medical scales, NTEP does not address the critical aspects of patient safety, efficacy, or performance that are unique to medical devices. It's like bringing a very precise kitchen scale to the operating room – it might measure accurately, but it was never designed for that environment.
The accuracy testing required by NTEP is often already covered, and often exceeded, by the stringent requirements of ISO 13485 and MET standards. Therefore, seeking separate NTEP certification for medical equipment can be redundant, add unnecessary costs, and potentially complicate the design and maintenance of devices without providing any additional medical-specific benefits. For us, prioritizing medical-specific certifications ensures that the equipment is not just accurate, but also safe and suitable for its intended medical purpose.
Here's a quick comparison to highlight the difference:
| Feature | ISO 13485 & MET Certification | NTEP Certification |
|---|---|---|
| Primary Focus | Quality management, patient safety, efficacy, performance, reliability | Accuracy for commercial trade (weighing and measuring devices) |
| Scope | Medical device design, manufacturing, installation, servicing | Specific device types (e.g., scales, fuel dispensers) for commerce |
| Risk Assessment | Mandatory for patient and user safety | Not a component |
| Regulatory Compliance | Critical for global medical device regulations | Relevant for legal metrology (weights and measures) |
| Relevance for Medical | Highly relevant and essential | Generally irrelevant; medical-specific standards are superior |
| Cost/Benefit | Provides comprehensive assurance for medical applications | Adds cost and complexity without specific medical benefits |
The Journey to Certification: From Manufacturer to Your Facility
The path from a manufacturer's blueprint to a fully certified medical equipment in your facility is a rigorous one, ensuring every step prioritizes patient safety and device integrity.

How Manufacturers Obtain Certification
For manufacturers, obtaining certifications like ISO 13485 and MET mark is an intensive process that integrates quality and safety into every stage of product development.
- Strict Design Controls: It begins with robust design controls, ensuring that the equipment is conceived with its medical purpose and potential risks in mind. This involves detailed planning, design input, output, review, verification, and validation.
- Risk Assessment: Manufacturers must conduct thorough risk assessments (often following standards like ISO 14971) throughout the product lifecycle. This identifies potential hazards to users and patients, and implements controls to mitigate those risks. Design guidelines for medical products heavily emphasize user and patient risk assessment.
- Process Validation: Every manufacturing process, from assembly to sterilization, is validated to ensure it consistently produces the intended results and maintains product quality.
- Technical Documentation: Extensive technical documentation is compiled, including design specifications, manufacturing procedures, quality control records, and post-market surveillance data. This "technical file" demonstrates compliance with all applicable standards.
- Third-Party Audits: For ISO 13485, manufacturers undergo independent third-party audits of their quality management system to verify adherence to the standard. Similarly, MET certification involves rigorous product testing by accredited laboratories to confirm safety and performance.
Only after successfully navigating these demanding steps can a manufacturer proudly claim their products are certified medical equipment.
The Role of Regulatory Bodies like the FDA
While ISO and MET provide a framework for quality and safety, regulatory bodies play a crucial role in overseeing the market entry and post-market surveillance of certified medical equipment. For instance, in the United States, the Food and Drug Administration (FDA), specifically its Center for Devices and Radiological Health (CDRH), is the primary regulatory authority.
The FDA classifies medical devices based on their risk level, from Class I (low risk, like bandages) to Class III (high risk, like pacemakers). The regulatory pathway for each class varies:
- 510(k) Clearance: For most Class II devices, manufacturers must demonstrate that their device is "substantially equivalent" to a legally marketed predicate device.
- Premarket Approval (PMA): Class III devices, which pose the greatest risk, require PMA – a much more rigorous process involving clinical trials to demonstrate safety and effectiveness.
Similar regulatory bodies exist worldwide, such as the European Medicines Agency (EMA) in Europe, Health Canada, and others. These bodies often collaborate through initiatives like the International Medical Device Regulators Forum (IMDRF) to harmonize regulatory requirements and promote the global accessibility and safety of medical equipment. This international cooperation helps ensure that high standards are maintained across borders, benefiting healthcare facilities and patients everywhere.
The Smart Choice: A Deep Dive into Recertified Medical Equipment
In an era where healthcare costs are continually on the rise, and budgets are stretched thin, acquiring new medical equipment can be a daunting challenge. This is where recertified medical equipment steps in as a smart, cost-effective, and environmentally friendly alternative. It allows healthcare facilities to access advanced technology, manage budget constraints, and even contribute to sustainability efforts by extending the life of valuable equipment. For those looking to understand the nuances of buying pre-owned equipment, we have a comprehensive guide: A Beginner’s Guide to Buying Refurbished Medical Equipment.
Defining the Terms: Refurbished vs. Used vs. Recertified
The world of pre-owned medical equipment can be a bit of a linguistic maze. Let's clarify the terms:
- "Used" or "Pre-owned": This generally refers to equipment sold "as-is." It has been previously owned and operated, but typically undergoes minimal to no repairs or inspections before resale. It often comes without a warranty, and its operational reliability can be uncertain.
- "Reconditioned" or "Refurbished": This category implies that the equipment has undergone some level of repair or cosmetic improvement. "Cosmetic repairs" might involve cleaning, repainting, or replacing external casings. "Functional repairs" mean internal components were fixed or replaced to restore basic operational capability. However, the extent of refurbishment can vary widely, and it might not always bring the equipment back to original manufacturer specifications.
- "Recertified": This is the gold standard of pre-owned equipment. Recertified medical equipment has undergone a rigorous, multi-point inspection, repair, and testing process by certified biomedical technicians. The goal is to restore the equipment to meet or even exceed original manufacturer (OEM) specifications for performance, accuracy, calibration, cleanliness, and safety. This process often includes replacing worn or near-end-of-life components. Crucially, recertified equipment comes with documentation and a warranty, giving us confidence in its dependability.
Key Advantages of Purchasing Recertified Equipment
Choosing recertified equipment offers compelling benefits, especially for facilities operating within tight budgets:
- Significant Cost Savings: This is often the primary driver. Recertified equipment is typically 30-70% less expensive than new models. This substantial saving does not compromise functionality, safety, or patient care, allowing us to allocate resources to other critical areas.
- Functionality and Safety on Par with New: Don't let the "pre-owned" label fool you. When properly recertified by expert technicians, the equipment performs identically to its new counterparts. It undergoes rigorous testing to ensure it meets or exceeds OEM standards.
- Extended Equipment Lifespan: Through the recertification process, worn components are replaced, and the unit is thoroughly serviced. This effectively gives the equipment a "second life," extending its operational lifespan and providing years of reliable service.
- Warranty Inclusion: Reputable recertifiers stand behind their work. Recertified medical equipment typically comes with a warranty on parts and labor for normal wear and tear, offering peace of mind similar to new equipment. Extended service plans are often available too.

What to Look for When Buying Refurbished and Certified Medical Equipment
When we're considering purchasing refurbished or certified medical equipment, especially recertified units, it's crucial to be discerning. Here's what we should look for:
- Supplier Reputation: Choose a supplier with a proven track record. Look for companies with strong industry experience and positive reviews. A quality reseller will be transparent about their processes and stand behind their products.
- Rigorous Refurbishment Process: Inquire about the specifics of their recertification process. Does it involve multi-point inspections? Are worn components replaced? Is it restored to OEM specifications? The more detailed and stringent the process, the better.
- Certified Biomedical Technicians: Ensure that the recertification work is performed by certified biomedical technicians. These professionals have the expertise and training to properly inspect, repair, and calibrate complex medical devices. Many are OEM-trained, ensuring adherence to manufacturer standards.
- Documentation and Traceability: Demand comprehensive documentation. This should include a full process report, a "biomed's bill of health," calibration reports, electrical safety testing reports, and a list of any parts replaced. This documentation confirms the equipment's condition and compliance. For examples of what this documentation looks like, check out: Recertification documentation examples.
- Warranty and Service Plans: Confirm the warranty coverage on both parts and labor. Understand the terms and duration. Also, ask about available extended service plans or annual maintenance options, which can significantly prolong the equipment's lifespan.
Special Considerations for EMS and Pre-Hospital Groups
For EMS, first responders, and pre-hospital transport groups, the stakes are incredibly high, and budgets are often exceptionally tight. Certified medical equipment is not just a preference; it's a necessity for saving lives in critical moments. Recertified equipment presents a particularly attractive solution for these groups.
Operating within tight budgets means every dollar counts. Purchasing recertified capital medical equipment like defibrillators, monitors, AEDs, ventilators, suction units, and stretchers can offer the same life-saving functionality as new equipment at a fraction of the cost. This allows EMS providers to equip more ambulances or replace outdated units without compromising on quality or safety.
Dependability in the field is paramount. Recertified equipment, when sourced from reputable suppliers and refurbished to OEM standards, offers the reliability needed in emergency situations. The rigorous testing ensures that these devices perform accurately and safely, even under demanding conditions. For EMS professionals, knowing their equipment is certified medical equipment means they can focus on patient care, not equipment failure. You can explore a range of EMS equipment suitable for these needs here: Example of EMS equipment available on MedIX.
Beyond the Device: Certifications for Suppliers and Professionals
Our commitment to certified medical equipment doesn't stop at the device itself. It extends to the entire ecosystem: the suppliers who provide the equipment and the professionals who maintain it. This holistic approach ensures end-to-end quality and reliability. For insights into managing your equipment and accreditation needs, explore: Equipment Management and Accreditation - MedIX.
Certifications for Medical Equipment Suppliers
A reliable supplier is the cornerstone of successful medical equipment procurement. We look for suppliers who demonstrate their commitment to quality through various certifications and accreditations.
Many jurisdictions, for example, require medical equipment service providers to be licensed. While specific requirements vary by region (e.g., the Ohio Board of Pharmacy licenses Home Medical Equipment Service Providers in Ohio), the principle is universal: regulatory bodies ensure that companies engaging in the sale, delivery, installation, maintenance, or demonstration of medical equipment meet certain standards. We can often verify such licenses through online portals provided by regulatory bodies: Verify a supplier's license.
Beyond governmental licensing, national and international accreditation bodies assess a supplier's operational quality, business practices, and adherence to industry standards. These accreditations signal that a supplier has voluntarily undergone rigorous evaluation and meets high benchmarks for service, quality management, and ethical conduct. For us, a verified supplier network, backed by such credentials, is essential for transparent and trustworthy transactions.
The Importance of Certified Professionals
Behind every piece of certified medical equipment is a team of skilled professionals. The expertise of these individuals is critical, particularly in the recertification process.
Organizations like the Board of Certification/Accreditation (BOC) offer certifications for healthcare professionals, such as the Certified Durable Medical Equipment (CDME) Specialist. While the CDME specifically focuses on durable medical equipment, it highlights the importance of specialized knowledge. More broadly, Certified Biomedical Equipment Technicians (CBETs) are vital. These are the experts who perform the intricate inspections, repairs, and calibrations on medical devices. Many are OEM-trained, meaning they have received direct instruction from the original equipment manufacturers, giving them unparalleled insight into the devices.
Their role in the recertification process cannot be overstated. It is their expertise that ensures a pre-owned device meets or exceeds manufacturer standards, making it dependable and safe for patient use. Without certified professionals, the integrity of the recertification process would be compromised, undermining the very concept of certified medical equipment.
Frequently Asked Questions about Certified Medical Equipment
We often encounter common questions about certified medical equipment. Here are some of the most frequent ones, answered directly:
What is the most important certification to look for in medical equipment?
The most important certifications to look for are ISO 13485 for quality management systems and the MET Mark (or equivalent safety certifications) for safety and performance. ISO 13485 assures us that the manufacturer has a robust quality system in place for medical devices, while MET Mark confirms the product itself has been tested for electrical safety, performance, and other critical aspects. These certifications ensure the device is designed, manufactured, and tested specifically for medical use, prioritizing patient safety and efficacy above all else.
Is recertified medical equipment as safe as new equipment?
Yes, when sourced from a reputable supplier, recertified medical equipment is considered as safe and effective as new equipment. This is because it undergoes a rigorous process by certified biomedical technicians to restore it to meet or exceed original manufacturer specifications. This process includes comprehensive testing for performance, accuracy, calibration, and electrical safety. Crucially, reputable recertified equipment comes with a warranty and detailed documentation of the work performed, providing the same level of assurance as a new purchase.
How do certifications impact the cost and efficiency of a healthcare facility?
Certifications significantly impact both the cost-effectiveness and operational efficiency of a healthcare facility. By ensuring high standards of quality and safety, certified medical equipment leads to:
- Reduced Long-Term Costs: Fewer equipment failures mean lower repair costs, less need for frequent replacements, and minimized downtime, which translates to sustained operational capability without unexpected expenses.
- Improved Operational Efficiency: Reliable, well-performing equipment allows healthcare professionals to deliver care smoothly and effectively, without interruptions caused by malfunctioning devices. This optimizes workflow and patient throughput.
- Ensured Regulatory Compliance: Using certified equipment helps facilities meet stringent regulatory requirements during audits, avoiding potential fines, penalties, or even loss of accreditation.
- Protection Against Liability: By demonstrating due diligence in procuring certified and safe equipment, facilities can protect themselves against potential liability issues arising from equipment-related incidents.
Certifications are an investment that pays off in reliability, safety, and operational stability.
Conclusion: Procuring Certified Equipment with Confidence
Navigating the landscape of certified medical equipment can seem complex, but with the right knowledge, it becomes a strategic advantage for any healthcare facility. We've seen that certifications like ISO 13485 and MET Mark are non-negotiable for ensuring patient safety, device performance, and operational efficiency. We've also learned that recertified equipment offers a smart, cost-effective alternative to new, provided it's sourced from reputable suppliers and backed by rigorous processes and warranties.
Understanding the distinctions between 'used,' 'refurbished,' and 'recertified' is crucial, as is recognizing the vital role of certified professionals and accredited suppliers. For us, making informed decisions means not just securing equipment, but securing peace of mind.
At MedIX, we understand these challenges. Our global B2B marketplace is designed to simplify procurement by connecting you with certified medical equipment suppliers. Through AI-matching, rigorous compliance checks, and reliable global logistics, we ensure verified equipment and transparent transactions worldwide. We empower you to make confident choices, knowing that every piece of equipment meets the highest standards.
Ready to equip your facility with confidence? Explore a wide range of certified medical equipment and find smarter procurement solutions with us: Explore a wide range of certified medical equipment.
Expert voices
Insights from leaders transforming medical equipment procurement






More from our blog
Discover the latest trends in medical technology and procurement
Stay ahead of medical technology
Get the latest insights, research, and market updates delivered straight to your inbox
Ready to transform your procurement
Discover how MedIX can streamline your medical equipment sourcing process



