Transforming medical equipment procurement globally

Your Gateway to the Global Healthcare Market
Global medical device distributors are specialized companies that bridge the gap between manufacturers and healthcare providers worldwide, handling everything from regulatory compliance and logistics to sales support and market access across multiple countries. As a procurement manager, choosing the right distributor partner can mean the difference between seamless supply chain operations and costly delays, compliance headaches, and frustrated clinical staff.
Key factors when evaluating global medical device distributors:
- Geographic reach - Coverage in your target markets (North America, Europe, Asia Pacific, etc.)
- Regulatory expertise - ISO 13485 certification, FDA compliance, CE marking knowledge
- Logistics capabilities - Warehousing infrastructure, cold chain management, reliable shipping
- Market access - Established relationships with hospitals and healthcare providers
- Value-added services - Product training, technical support, clinical education
- Financial stability - Proven track record and sustainable business model
- Compliance verification - Rigorous quality management systems and documentation
The global medical device market is projected to reach $600 billion by 2025, with the distribution sector growing at a CAGR of 5.5% from 2020 to 2027. This explosive growth is driven by an aging population, rising chronic disease prevalence, and rapid technological advancement in medical devices. For an overview of how authorities classify and regulate devices, you can refer to resources such as the World Health Organization's page on medical devices.
But here's the challenge: navigating this complex landscape requires more than just finding a company that can ship products. You need a strategic partner who understands local regulations, maintains reliable supply chains, and provides the support your clinical teams need to improve patient outcomes.
The right distributor doesn't just move boxes. They provide market intelligence, manage regulatory problems across borders, offer clinical training to healthcare providers, and create the feedback loops that help manufacturers improve their products. Some companies supply devices to over 70 nations, while other firms have experience in more than 70 countries, demonstrating the truly global nature of this industry.
Whether you're a healthcare institution seeking verified suppliers or a manufacturer looking to expand internationally, understanding how to identify and partner with the right distributor is essential for success in today's connected healthcare ecosystem.
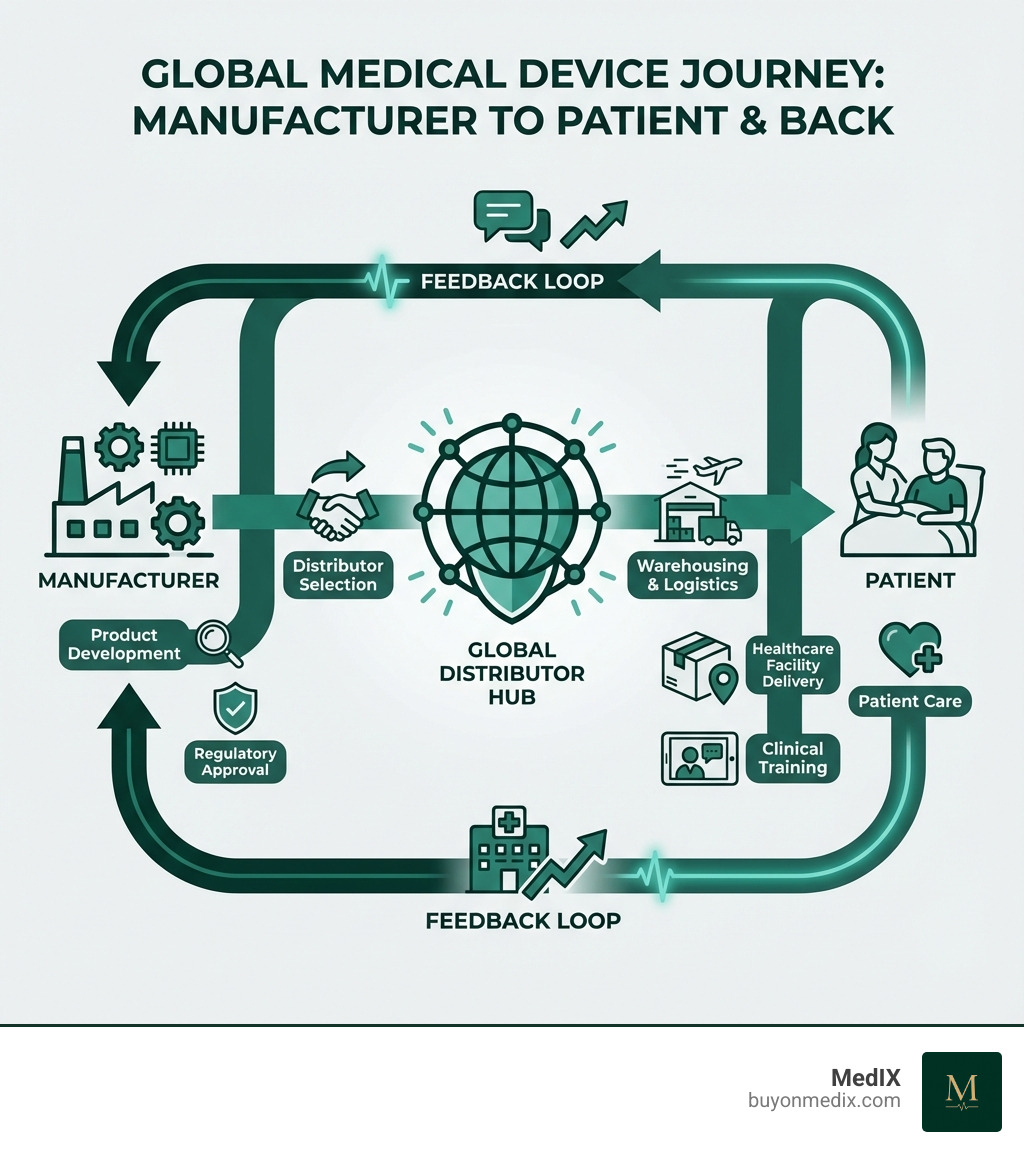
The Crucial Role of Distributors in the Healthcare Ecosystem
Global medical device distributors are the unsung heroes of the healthcare supply chain. They ensure that cutting-edge medical technologies, life-saving equipment, and essential supplies reach the hospitals, clinics, and healthcare providers who need them most. Their role extends far beyond simple logistics; they are instrumental in market expansion, supply chain management, sales and marketing support, and ultimately, in improving patient outcomes and operational efficiency within healthcare facilities.
Bridging the Gap: From Manufacturer to End-User
Imagine a brilliant medical device manufacturer with a product, but no way to get it to a hospital in a different country. That's where distributors step in! They act as crucial intermediaries, enabling manufacturers to expand their reach into new international healthcare markets without having to build extensive infrastructure in every region. Some distributors have extensive experience helping companies enter and expand in over 70 countries, operating in key regions including North & South America, Europe, Middle East & Africa, and Asia Pacific, demonstrating the global networks they establish.
Distributors overcome geographical barriers by establishing robust supply chain networks. They manage the complex logistics of international shipping, customs, and local delivery. Large-scale distributors, for instance, may boast millions of square feet of warehousing and offer next-day shipping to a vast majority of their customers – a testament to the scale and efficiency required. For specialized markets, experienced distributors provide direct market access to hospitals, leveraging over 20 years of experience in medical device distribution and logistics in a specific region. This ensures that even specialized devices, like those for interventional radiology or cardiology, reach their intended users efficiently.
More Than Logistics: Value-Added Services That Make a Difference
Distributors do much more than just move products from point A to point B. They are strategic partners offering a suite of value-added services. This includes comprehensive product training for healthcare providers, ensuring that medical staff know how to correctly and safely operate new equipment. They provide technical support, helping to troubleshoot issues and maintain devices. Post-sales service is also critical, contributing to the longevity and effective use of medical equipment.
Furthermore, distributors often bring invaluable clinical practice expertise, acting as a bridge between manufacturers and the end-users. They gather feedback from healthcare professionals, creating a vital loop that helps manufacturers refine and improve their products. Some distributors provide comprehensive support for marketing new products and expanding existing ones into diverse global markets, including digital and in-person marketing needs, and attending global conventions. This proactive engagement makes them an indispensable part of the healthcare ecosystem.
Impact on Patient Care and Hospital Operations
The efficient operation of global medical device distributors directly translates to better patient care and more streamlined hospital operations. By managing the supply chain effectively, distributors ensure the timely access to essential devices and supplies. This is crucial for emergency situations, scheduled surgeries, and ongoing patient care.
Distributors also assist healthcare providers with inventory management, often offering sophisticated inventory management solutions which help control stock, reduce waste, and ensure products are available when needed. This focus on operational efficiency helps healthcare facilities run smoother, allowing medical professionals to focus on what they do best: caring for patients. By providing customized solutions and ensuring access to the latest technologies, distributors play a vital role in improving patient outcomes and enhancing the overall quality of care.
A Strategic Approach to Selecting Global Medical Device Distributors
Choosing the right global medical device distributors is a critical decision for manufacturers aiming for international expansion. It's not just about finding someone to sell your products; it's about forming a strategic partnership based on trust, shared goals, and mutual benefit. This requires careful due diligence, a deep understanding of market dynamics, and a clear vision for collaboration.
Step 1: Defining Your Ideal Distributor Profile
Before starting on the search, we must first define what our ideal distributor looks like. This involves considering several key factors:
- Geographic Focus: Where do we want to sell our products? North America is the largest market for medical device distributors, followed by Europe and Asia Pacific. However, other regions like the Middle East, Africa, and South America also present significant opportunities. Some distributors operate across all these continents, offering broad reach, while some manufacturers actively seek distributorship in regions like Oceania, the Middle East, CIS Nations, South America, North America, Europe, Africa, and Asia. This shows the diverse needs and opportunities in global markets.
- Product Specialization: Does the distributor have expertise in our specific medical device category? Some distributors specialize in interventional devices, while others focus on cardiology, orthopedics, or sterile processing. For instance, some may focus on niches like Aesthetic & Reconstructive, ENT, Ophthalmology, Single-Use Instruments, and Sterile Processing, while others cover a wide range including cardiology, neuromodulation, and endoscopy. Matching our product's niche with a distributor's specialization ensures they have the right connections and knowledge.
- Required Services: Beyond basic distribution, what additional support do we need? This might include regulatory guidance, marketing and sales support, training, or post-market surveillance.
Step 2: The Vetting Process for Global Medical Device Distributors
Once we have a clear profile, the rigorous vetting process begins. This is where we separate the good from the great, ensuring our partners are reliable and capable.

Our vetting checklist should include:
- Background Checks and Reference Checks: Speak to their current and past partners to understand their performance, reliability, and ethical standards.
- Reviewing Compliance History: This is paramount in the medical device industry. We must ensure they have a spotless record with local regulatory bodies.
- Certifications: Look for internationally recognized certifications like ISO 13485, which demonstrates a robust quality management system. Many reputable distributors highlight their adherence to ISO 13485, along with CE Marking and FDA approval where applicable. Some also provide legal and regulatory consultation for complex markets.
- Assessing Market Reputation: A strong reputation among healthcare providers and within the industry is a significant asset.
- Financial Stability: A financially sound partner ensures they can invest in inventory, marketing, and necessary infrastructure.
Step 3: Establishing and Managing a Successful Partnership
The relationship doesn't end once a distributor is selected; it's just the beginning. Establishing clear distribution agreements is essential, outlining terms, responsibilities, and performance expectations. We need to set measurable Key Performance Indicators (KPIs) to track success, such as sales targets, market penetration, and customer satisfaction.
Open and consistent communication channels are vital for a healthy partnership. Regular performance reviews allow us to assess progress, address challenges, and adapt strategies as needed. Some companies even offer distributor management services, taking on the responsibility of overseeing the distributor network on behalf of manufacturers with limited resources. This ongoing management ensures the partnership remains fruitful and aligned with our global expansion goals.
Navigating the Complexities: Key Challenges in Global Distribution
The world of global medical device distributors is dynamic and rewarding, but it’s certainly not without its problems. These challenges require careful planning, robust systems, and agile strategies to overcome.
One of the most significant challenges is regulatory compliance. Each country and region has its own unique set of regulations for medical devices, which can be incredibly complex and time-consuming to steer. What's approved in one market might require extensive additional documentation or testing in another. Distributors play a crucial role in understanding and adhering to these diverse international market requirements, from FDA approval in the U.S. to CE Marking in Europe, and specific national standards elsewhere. Without expert guidance, manufacturers can face significant delays or even market exclusion.
Logistics problems and supply chain disruptions are ever-present concerns. Geopolitical events, natural disasters, and even pandemics can quickly halt the movement of goods. Distributors must maintain resilient supply chains, with contingency plans for unforeseen circumstances. This includes managing warehousing across different climates, ensuring proper cold chain management for sensitive products, and dealing with customs complexities in various ports.
Inventory control is another critical area. Balancing adequate stock levels to meet demand without incurring excessive holding costs or risking expiration is a fine art. Distributors use sophisticated inventory management systems to optimize stock levels and ensure timely delivery to healthcare providers.
Furthermore, price pressures from healthcare systems and intense competition mean that distributors must constantly seek efficiencies while maintaining high service quality. Finally, the rapid pace of technological adoption means distributors must continuously update their own systems and expertise to keep pace with innovations in medical devices themselves and in logistics technology.
The Future of Distribution: Technology and Emerging Trends
The landscape of global medical device distributors is undergoing a profound change, driven largely by technological advancements and evolving market demands. We are witnessing an exciting shift towards more integrated, efficient, and data-driven distribution models.
How Technology is Reshaping the Role of Global Medical Device Distributors
Technology is not just an add-on; it's becoming the backbone of modern medical device distribution.
- Inventory Automation: Robotic process automation (RPA) and automated storage and retrieval systems (AS/RS) are revolutionizing warehouses. For example, some distributors are collaborating with logistics automation specialists for new automated storage installations, demonstrating the move towards highly automated distribution centers. This technology significantly improves efficiency, accuracy, and speed in managing vast inventories of medical supplies.
- Real-time Tracking and Predictive Analytics: Advanced software allows for real-time tracking of shipments, providing transparency and predictability. Data analytics tools help distributors forecast demand, optimize routing, and identify potential supply chain bottlenecks before they occur.
- Barcode Scanning Systems: Advanced barcode scanning systems streamline inventory management for healthcare providers, reducing human error and improving efficiency in ordering and stock control.
- Cloud-based Platforms and AI: Cloud-based platforms and AI are being leveraged to automate inter-organizational processes in the medical device sector. This includes AI-matching for connecting buyers with verified manufacturers and streamlining import/export requirements, making global trade more accessible and efficient. This digital change allows for seamless communication and data exchange across the entire supply chain.
Key Trends to Watch in the Distribution Landscape
As we look ahead, several key trends are shaping the future of medical device distribution:
- Direct-to-Patient Models: With the rise of home healthcare and telemedicine, we may see more medical devices being distributed directly to patients' homes, requiring new logistics and support structures.
- Value-Based Healthcare Partnerships: Distributors are increasingly focusing on delivering improved clinical, financial, and operational outcomes for their customers, rather than just selling products. This means combining scale and agility to improve healthcare delivery and closer collaboration with healthcare providers to achieve shared goals, including reducing hospital readmissions and enhancing quality of care.
- Focus on Sustainability: Environmental responsibility is becoming a critical factor. Some distributors, for example, have committed to becoming net zero by 2040, highlighting a growing trend towards sustainable practices in the supply chain.
- Increased Data Security: As more data is collected and exchanged digitally, ensuring robust cybersecurity measures to protect sensitive patient and commercial information will be paramount.
These trends underscore the evolving nature of global medical device distributors, positioning them as technology-forward, outcome-focused partners in the ever-changing healthcare ecosystem.
Frequently Asked Questions about Medical Device Distribution
We often encounter common questions when discussing the intricate world of global medical device distributors. Let's address some of the most pressing ones.
How do distributors ensure regulatory compliance in different countries?
Ensuring regulatory compliance is arguably one of the most critical and challenging aspects of international medical device distribution. Our distributors employ a multi-faceted approach:
- Local Expertise: They possess in-depth knowledge of local laws, regulations, and cultural nuances in each market. This often involves having dedicated regulatory teams or consultants within those regions. For instance, some offer legal and regulatory consultation specifically for markets known for their unique complexities.
- Quality Management Systems (QMS): Adherence to international standards like ISO 13485 is fundamental. This certification demonstrates a commitment to quality and compliance throughout the entire distribution process. Many reputable distributors highlight their ISO 13485 certification.
- Tracking Specific Regulations: Distributors carefully track and comply with regional requirements such as FDA approval (United States), CE Mark (European Union), Health Canada regulations, PMDA (Japan), and other national medical device agencies. Experienced distributors provide market access and regulatory support across dozens of countries, demonstrating the breadth of this expertise.
- Documentation and Auditing: They maintain meticulous records for traceability, adverse event reporting, and facilitate regular audits by manufacturers and regulatory bodies.
What are the first steps to becoming an international medical device distributor?
For companies aspiring to join the ranks of global medical device distributors, the journey begins with strategic preparation:
- Develop Market Expertise: Deeply understand the healthcare landscape, unmet needs, and competitive environment in your target regions. What medical devices are in demand? What are the local purchasing processes?
- Build a Robust Logistics Network: Establish secure warehousing facilities, cold chain capabilities if needed, and efficient transportation routes. This includes understanding customs procedures and import/export regulations.
- Understand Regulatory Frameworks: Invest in expertise to steer the complex regulatory requirements of the countries you plan to operate in. This is non-negotiable for medical devices.
- Secure Funding: International distribution requires significant capital for inventory, infrastructure, and operational costs.
- Establish Manufacturer Relationships: Actively seek out medical device manufacturers looking for international partners. Some manufacturers actively invite inquiries from potential international distributors and list specific regions where they are open to distributorship, such as Oceania, the Middle East, and South America. Building a portfolio of high-quality products is key.
What are the different types of medical device distribution models?
The medical device industry uses several distribution models, each with its own advantages and disadvantages:
- Direct Sales: The manufacturer sells directly to healthcare providers, often through their own sales force. This model offers maximum control over sales and marketing but requires significant investment in infrastructure and personnel in each market. A manufacturer might use direct sales representatives in the U.S.
- Third-Party Distributors: An independent company handles sales, marketing, logistics, and sometimes regulatory compliance in a specific region or country. This is a common model for international expansion, leveraging the distributor's local expertise and existing networks. Many manufacturers rely on and partner with third-party distributors.
- Exclusive Agreements: The manufacturer grants a single distributor the sole right to sell their products within a defined territory. This can lead to greater commitment from the distributor.
- Non-Exclusive Agreements: The manufacturer can work with multiple distributors in the same territory, fostering competition but potentially diluting focus.
- Hybrid Models: Many manufacturers use a combination of these approaches. For example, they might use direct sales in their home market or key strategic regions, while relying on third-party distributors for other international markets. A manufacturer might employ a hybrid model with U.S. direct sales and international partners.
Here's a quick comparison:
| Feature | Direct Sales Model | Third-Party Distribution Model |
|---|---|---|
| Control | High | Moderate to Low |
| Investment Required | High (infrastructure, staff, regulatory) | Low (leverages distributor's existing resources) |
| Market Access Speed | Slower (requires building presence) | Faster (distributor has established network) |
| Local Expertise | Built internally over time | Immediate (distributor has existing knowledge) |
| Risk | Higher (direct exposure to market fluctuations) | Lower (shared with distributor) |
| Profit Margin Potential | Higher (no distributor cut) | Lower (distributor takes a cut) |
| Suitability | Large companies, high-volume products, strategic markets | Smaller companies, niche products, rapid international expansion |
Conclusion: Finding Your Perfect Partner in a Connected World
In the dynamic and rapidly growing global medical device market, the role of global medical device distributors is more critical than ever. They are not merely logistical conduits but strategic partners who facilitate market access, ensure regulatory compliance, provide essential support to healthcare providers, and ultimately contribute to improved patient outcomes worldwide. The market's projected growth to $600 billion by 2025 underscores the immense importance of these partnerships.
For both manufacturers seeking to expand their global footprint and healthcare providers looking for reliable access to verified medical equipment, the process of finding the right distributor demands a strategic approach. This involves meticulous due diligence, a clear understanding of market needs, and a commitment to fostering strong, transparent relationships. Technology is continuously reshaping this landscape, making distribution more efficient, data-driven, and responsive to global demands.
At MedIX, we understand these complexities. Our platform is designed to simplify procurement by connecting certified medical equipment suppliers with hospitals and clinics worldwide. We leverage AI-matching, rigorous compliance checks, and reliable global logistics to ensure you find verified equipment and experience transparent transactions, no matter where you are in the world.
Whether you're a manufacturer looking to streamline your distribution network or a healthcare provider aiming to source the best medical devices, partnering with the right entity is your gateway to success in this connected world.
Expert voices
Insights from leaders transforming medical equipment procurement






More from our blog
Discover the latest trends in medical technology and procurement
Stay ahead of medical technology
Get the latest insights, research, and market updates delivered straight to your inbox
Ready to transform your procurement
Discover how MedIX can streamline your medical equipment sourcing process



