Transforming medical equipment procurement globally

Why Medical Device Certification Is the Foundation of Healthcare Safety

Medical device certification is the formal process by which regulatory authorities verify that a medical device meets defined safety, quality, and performance standards before it can be legally marketed and used. For procurement managers sourcing equipment for global healthcare institutions, understanding this process is essential because it directly impacts patient safety, legal compliance, and your ability to access different markets.
Quick Overview: What Medical Device Certification Means
- Safety Assurance: Confirms devices will not harm patients or healthcare providers when used as intended
- Market Access: Required for legal distribution in the US, EU, and most global markets
- Quality Standards: Ensures consistent manufacturing and performance
- Regulatory Compliance: Protects your institution from legal and financial risks
- Performance Verification: Validates that devices work as claimed
The stakes are high. The FDA is responsible for assuring medical devices available in the United States are safe and effective throughout their total product lifecycle. Similarly, the European Medicines Agency (EMA) offers clinical advice to manufacturers of high-risk medical devices through expert panels. These regulatory bodies do more than check boxes; they act as a safeguard between patients and potentially dangerous equipment.
For procurement managers, certification is not just a regulatory hurdle. It is a first line of defense when vetting suppliers. A properly certified device means someone has already verified technical documentation, reviewed clinical data, audited manufacturing processes, and ensured ongoing quality controls.
Federal law established a risk-based device classification system with three classes (I, II, and III), each requiring different levels of regulatory control. Class I devices face general controls, Class II devices require special controls and often a 510(k) premarket notification, and Class III devices, the highest risk, demand rigorous Premarket Approval (PMA).
The landscape is evolving as well. The FDA issued the Quality Management System Regulation (QMSR) Final Rule, which amends device manufacturing requirements by incorporating ISO 13485:2016, effective February 2, 2026. This harmonization with international standards affects how manufacturers prove compliance and how you evaluate their certifications.
Understanding what sits behind that certification seal, including testing, documentation, audits, and ongoing surveillance, helps you make informed sourcing decisions, reduce supply chain risks, and ensure your institution receives equipment that meets the standards patients deserve.
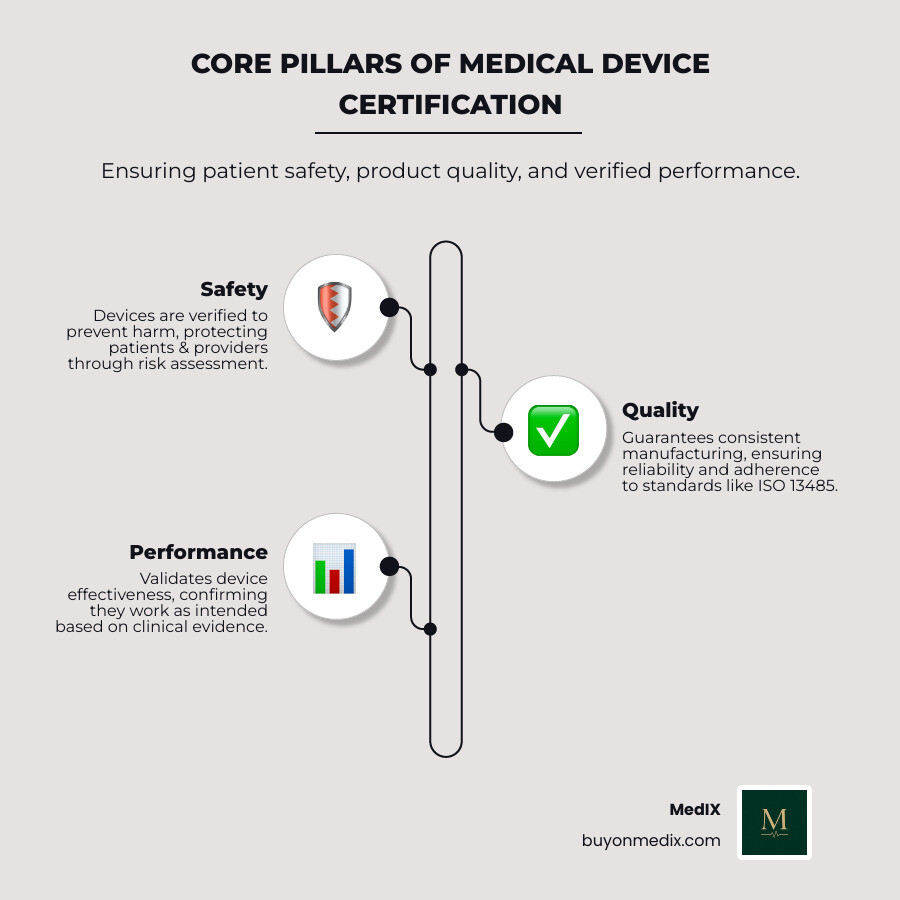
Why Certification Matters: Ensuring Safety and Opening up Markets
Imagine a world where medical devices could be sold without any checks or balances. This is precisely why medical device certification is not just a bureaucratic formality; it is the bedrock of patient safety and a non-negotiable gateway to global markets. For procurement managers, understanding its impact is crucial.
Certification builds patient and provider confidence. When a device carries a recognized certification mark, it signals that it has undergone evaluation and meets defined safety and performance criteria. This trust is vital in healthcare, where clinical teams rely on equipment every day.
Certification is also a powerful tool for risk mitigation. The process identifies potential hazards, evaluates clinical performance, and checks manufacturing consistency. By sourcing certified medical equipment, you reduce the likelihood of device failures, adverse events, and costly recalls, protecting both patients and institutions from legal and financial risks. For more detail on what makes equipment certified, see Certified Medical Equipment.
From a business perspective, medical device certification provides a competitive advantage. In a crowded marketplace, certified products stand out by demonstrating a manufacturer's commitment to quality and compliance. This supports market access and reinforces a brand's reputation for reliability.

The Impact of CE Marking and FDA Approval
When discussing global market access for medical devices, two acronyms often dominate: CE Marking and FDA clearance or approval. These are not just stamps on a product; they are regulatory pathways that determine whether a device can be legally sold in major markets such as the European Union and the United States.
CE Marking signifies that a product conforms with European health, safety, and environmental protection standards. For medical devices, this means compliance with the EU Medical Device Regulation (MDR) (Regulation (EU) 2017/745). Obtaining CE Marking is essential for manufacturers aiming to sell their devices across the 27 EU member states, as well as in Iceland, Liechtenstein, and Norway. It is a declaration by the manufacturer that their product meets the applicable requirements. For more details, see CE Marked Medical Devices.
For the U.S. market, devices must obtain FDA clearance or approval. The FDA, through its Center for Devices and Radiological Health (CDRH), ensures that medical devices are safe and effective. The type of submission required, such as 510(k) clearance or Premarket Approval (PMA), depends on the device's classification and associated risk. Both CE Marking and FDA authorizations are central to global market entry and help build trust with regulators and healthcare providers.
The Role of Third-Party Organizations
While regulatory bodies set the rules, they often rely on specialized third-party organizations to assist with the certification process.
In the EU, these organizations are known as Notified Bodies. They are designated by national authorities to assess the conformity of certain medical devices before they can be placed on the market. Their responsibilities include reviewing manufacturers' technical documentation, conducting quality system audits (for example to ISO 13485 standards), and reviewing clinical evaluations. Without a Notified Body's positive assessment, many medical devices cannot receive CE Marking. Companies like DNV and TÜV SÜD are examples of Notified Bodies that offer medical device certification and compliance services. You can learn more at Medical device certification & compliance - DNV.
In the U.S., the FDA generally conducts its own reviews but does use Accredited Persons for certain 510(k) submissions. These third parties can conduct primary reviews of specific device types, which may streamline the clearance process. This independent verification adds an extra layer of scrutiny, helping ensure that devices meet the necessary technical, safety, and quality standards before reaching patients.
The Gatekeepers: An Overview of Key Regulatory Bodies
Navigating the global medical device market can feel like dealing with multiple jurisdictions, each with its own gatekeepers and rules. For procurement teams, it helps to understand who the key regulatory bodies are and what their mandates involve. While the regulatory landscape is broad, the U.S. Food and Drug Administration (FDA) and the European regulatory framework (including the European Medicines Agency and Notified Bodies) are among the most influential.
These authorities exist primarily to safeguard public health by ensuring medical devices are safe, effective, and of high quality.
The U.S. Food and Drug Administration (FDA)
The FDA is the primary regulatory body for medical devices in the United States. Its mission is to assure that medical devices available in the U.S. are safe and effective throughout their total product lifecycle. The Center for Devices and Radiological Health (CDRH) is the branch within the FDA responsible for this oversight.
The FDA employs a risk-based classification system for medical devices, categorizing them into Class I, Class II, and Class III. This classification dictates the level of regulatory control required.
- Class I devices (low risk) are subject to general controls.
- Class II devices (moderate to high risk) require general controls and special controls, and often a Premarket Notification (510(k)) submission to demonstrate substantial equivalence to a legally marketed device.
- Class III devices (high risk) are subject to general controls and the most stringent pathway, Premarket Approval (PMA), which often requires clinical data.
Beyond premarket review, the FDA emphasizes post-market surveillance to monitor device safety and effectiveness once products are in use. This includes adverse event reporting and quality system audits. For a detailed explanation of how the FDA regulates devices, see the Overview of Device Regulation.
A notable development is the FDA's Quality Management System Regulation (QMSR) Final Rule, effective February 2, 2026. This rule harmonizes the FDA's device current good manufacturing practice (CGMP) requirements (21 CFR Part 820) with the international standard ISO 13485:2016. As a result, U.S. manufacturers will increasingly align with global quality management system standards.
The European Regulatory Framework (EMA & Notified Bodies)
In Europe, the regulatory landscape is governed by the Medical Device Regulation (MDR) (Regulation (EU) 2017/745). This framework replaced older directives and introduced more stringent requirements for medical devices.
The European Medicines Agency (EMA) plays a role, particularly for high-risk devices. The EMA offers scientific advice to manufacturers of Class III medical devices and certain Class IIb active medical devices through expert panels. This advice focuses on clinical development strategies and clinical investigation proposals, as outlined in Article 61(2) of the MDR. Manufacturers can also request Joint Scientific Consultations to receive parallel advice from EMA expert panels and the Health Technology Assessment Coordination Group.
Notified Bodies are central to the European system. Unlike the FDA, which directly reviews most submissions in the U.S., the EU relies on these third-party organizations to assess the conformity of devices with the MDR. Their responsibilities include reviewing technical documentation, auditing manufacturers' quality management systems, and verifying that clinical evaluations are adequate. Without a positive assessment from a Notified Body, most moderate to high-risk devices cannot receive CE Marking, which is the manufacturer's declaration that a product complies with the essential requirements of the MDR. For more background, refer to CE Marked Devices.
Navigating the Path to Compliance: The Medical Device Certification Process
The journey from a medical device concept to market approval is a structured process. With a clear view of the main steps, procurement teams can better understand what sits behind each certificate.
At each stage, manufacturers must demonstrate that their device is safe, performs as intended, and adheres to applicable quality standards. This spans design controls and risk assessments, manufacturing processes, labeling, and post-market surveillance plans.
Key Differences in Medical Device Certification for Class I, II, and III Devices
A core aspect of medical device certification is risk-based classification, because it determines the regulatory pathway. U.S. federal law (Federal Food, Drug, and Cosmetic Act, section 513) established a system that groups devices into Class I, II, and III based on their potential risk to patients.
Class I Devices (Low Risk): These devices generally pose the lowest risk. Examples include manual stethoscopes and tongue depressors. They are primarily subject to general controls, such as proper labeling, establishment registration, and device listing. Many Class I devices are exempt from premarket notification requirements.
Class II Devices (Moderate to High Risk): These devices are more complex and pose a higher risk than Class I. Examples include infusion pumps and powered wheelchairs. In addition to general controls, Class II devices require special controls to help ensure safety and effectiveness. Most Class II devices require a Premarket Notification (510(k)) submission, demonstrating that the device is substantially equivalent to a legally marketed predicate device.
Class III Devices (High Risk): These are the highest-risk devices, often life-sustaining, life-supporting, implanted, or those that present a potential unreasonable risk of illness or injury. Examples include pacemakers and implantable defibrillators. Class III devices are subject to general controls and require Premarket Approval (PMA), the most stringent regulatory pathway, which typically demands extensive clinical data.
For a deeper overview of how devices are classified, refer to the Classification of Medical Devices PDF and the FDA explanation of Regulatory Controls for medical devices.
Understanding the Role of Quality Management Systems in Medical Device Certification
Behind every certified medical device is a Quality Management System (QMS). A QMS provides the structure that ensures consistency, safety, and performance from conception to post-market activities.
The international reference standard for medical device QMS is ISO 13485:2016 Medical devices – Quality management systems – Requirements for regulatory purposes. It specifies requirements for a QMS where an organization needs to demonstrate its ability to provide medical devices and related services that consistently meet customer and regulatory requirements. It covers design controls, risk management, manufacturing processes, complaint handling, and corrective and preventive actions.
The FDA issued the Quality Management System Regulation (QMSR) Final Rule, which amends the device current good manufacturing practice (CGMP) requirements of the Quality System (QS) regulation (21 CFR Part 820) by incorporating ISO 13485:2016 by reference, effective February 2, 2026. This alignment is intended to make it easier for manufacturers operating globally to meet both U.S. and international expectations. For details on the FDA's QMS requirements, see the FDA's Quality System (QS) Regulation/Medical Device Current Good Manufacturing Practices (CGMP).
For buyers, a supplier's adherence to ISO 13485 and preparedness for QMSR is a strong indicator of their commitment to quality.
Modern Challenges: Software, Cybersecurity, and Data Privacy
The rapid evolution of technology has added new dimensions to medical device certification, particularly around software, cybersecurity, and data privacy.
Software as a Medical Device (SaMD) and other digital health components, such as mobile medical apps, wireless devices, and AI or machine learning in SaMD, require specific regulatory scrutiny. Certification for these products involves evaluating the software development lifecycle, risk management for software failures, and usability.
Cybersecurity is now a central concern. Connected devices can be targets for cyberattacks that might compromise patient data or device performance. Certification processes increasingly expect robust cybersecurity risk management, including threat modeling, vulnerability assessment, and plans for security updates. The FDA has issued guidance on cybersecurity expectations for networked medical devices.
Data privacy is another critical consideration. Medical devices often collect, store, and transmit sensitive patient health information. Compliance with regulations such as HIPAA in the U.S. and GDPR in Europe is essential. Manufacturers must show that devices incorporate privacy by design, including appropriate data encryption, access controls, and secure data handling. Certification reviews take these elements into account.
Global Harmonization and Ongoing Compliance
The global nature of the medical device industry has driven efforts to harmonize regulatory requirements. This supports a consistent standard of safety and quality worldwide and can simplify compliance for manufacturers that sell into multiple regions.
Initiatives like the International Medical Device Regulators Forum (IMDRF) and the Medical Device Single Audit Program (MDSAP) are central to this work. The IMDRF brings together regulators from various jurisdictions to promote convergence of regulatory requirements. MDSAP, a program under the IMDRF, allows a single audit of a medical device manufacturer's quality management system to satisfy the requirements of multiple regulatory authorities (Australia, Brazil, Canada, Japan, and the U.S.).
As noted earlier, the FDA's QMSR Final Rule, effective February 2, 2026, incorporates ISO 13485:2016. This move aligns U.S. quality system requirements with the international standard already recognized by many other regulatory bodies, including those in the EU.
Below is a simplified comparison of key requirements for U.S. (FDA) and EU (CE) markets to illustrate the general alignment.
| Aspect | U.S. (FDA) | EU (CE/MDR) |
|---|---|---|
| Core legal framework | Federal Food, Drug, and Cosmetic Act; 21 CFR Parts 807, 812, 814, 820 | Medical Device Regulation (MDR) (Regulation (EU) 2017/745) |
| Market authorization mark | No physical mark from FDA; clearance/approval documented by FDA database | CE Marking affixed to device and packaging |
| Risk classification | Class I, II, III (risk-based) | Class I, IIa, IIb, III (risk-based) |
| Premarket pathways | 510(k), De Novo, PMA, Investigational Device Exemption (IDE) | Conformity assessment routes under MDR with Notified Body where required |
| QMS requirements | 21 CFR Part 820; QMSR aligning with ISO 13485:2016 | ISO 13485 widely used; MDR requires a QMS and Notified Body assessment for most higher-risk devices |
| Clinical evidence | Required based on risk and pathway; guidance via FDA and CDRH | Clinical evaluation and, where appropriate, clinical investigation under MDR |
| Post-market activities | Medical Device Reporting (MDR), corrections and removals, post-market studies | Vigilance reporting, Post-Market Surveillance (PMS), Post-Market Clinical Follow-up (PMCF) |
Common Challenges and How to Prepare
Manufacturers often face similar problems on the road to medical device certification.
Common challenges include:
- Incomplete or inconsistent technical documentation
- Insufficient clinical evidence for the intended indications
- Evolving regulations that change expectations mid-project
- Unanticipated testing failures or usability issues
Practical ways to streamline certification:
- Start regulatory planning early and align design decisions with requirements
- Engage regulatory and clinical experts, especially for higher-risk devices
- Maintain clear, organized documentation throughout development and production
- Build change control and risk management into the QMS from the outset
Post-Market Surveillance: The Work Does Not End at Certification
Certification is a milestone, not an endpoint. Once devices are on the market, manufacturers must track how products perform in real-world use and respond to new information.
Key post-market activities include:
- Adverse event reporting to regulators
- Post-market clinical follow-up (PMCF) or similar studies where required
- Regular audits and internal reviews of the quality system
- Labeling and instructions for use updates when new risks or mitigations are identified
Staying informed about evolving rules and expectations is essential. For updates on regulatory developments relevant to buyers and suppliers, see Medical Regulatory Updates.
Expert voices
Insights from leaders transforming medical equipment procurement






More from our blog
Discover the latest trends in medical technology and procurement
Stay ahead of medical technology
Get the latest insights, research, and market updates delivered straight to your inbox
Ready to transform your procurement
Discover how MedIX can streamline your medical equipment sourcing process



