Transforming medical equipment procurement globally

Why CE Marked Devices Are Your Gateway to European Healthcare Markets
CE marked devices are medical products bearing the CE (Conformité Européenne) mark, which indicates they meet European Union (EU) standards for safety, health, and environmental protection. This mark is a legal prerequisite for selling medical devices across the European Economic Area (EEA).
Key facts about CE marked devices:
- Market Access: Enables sale across all 27 EU member states, plus Iceland, Norway, and Liechtenstein.
- Consumer Base: Provides access to over 500 million potential consumers.
- Legal Requirement: Mandatory for medical devices before they can be placed on the European market.
- Manufacturer Declaration: Represents the manufacturer's formal declaration that the device complies with all applicable EU regulations.
- Not a Quality Mark: Signifies regulatory compliance, not product quality or performance superiority.
- Regulatory Framework: Governed by the Medical Device Regulation (EU) 2017/745 (MDR).
For procurement managers, understanding CE marking is essential. It is proof that a manufacturer has completed rigorous conformity assessments and assumes full responsibility for the product's safety and performance. The mark is either a self-declaration by the manufacturer (for low-risk devices) or a certification by an independent Notified Body (for higher-risk devices). Without it, medical devices cannot legally enter the European market.
What is CE Marking and Why is it Essential for Medical Devices?
The CE mark, short for "Conformité Européenne," is a manufacturer's declaration that a product meets the EU's health, safety, and environmental protection requirements. It acts as a product's passport, allowing it to be marketed freely throughout the EEA, regardless of its country of origin. It is not a quality seal but a signal of regulatory compliance.
For medical devices, CE marking is a cornerstone of public health for several key reasons:
- Legal Mandate: The Medical Devices Regulation (EU) 2017/745 (MDR) makes CE marking a non-negotiable legal requirement. Without it, a device cannot be sold in the EU.
- Patient Safety: The process ensures devices meet stringent requirements for safety and performance, verifying that they perform as intended without posing undue risks. This gives procurement managers confidence that a device has been vetted to deliver reliable clinical outcomes.
- Market Access: It provides a unified pathway to a market of over 500 million consumers, removing technical trade barriers and eliminating the need for separate approvals in each member state. You can find official guidance on European market access for medical devices.
- Building Trust: The CE mark assures healthcare professionals and patients that a device meets fundamental safety and performance standards, which is invaluable in a field where equipment reliability directly impacts lives.
The Manufacturer's Role and Responsibilities
For CE marked devices, the manufacturer is at the center of the process, with responsibilities spanning the device's entire lifecycle. This commitment to continuous regulatory compliance is fundamental.
Who is Responsible for Obtaining CE Marking?
The manufacturer is legally responsible for ensuring their product meets all EU requirements and for affixing the CE mark, even if parts of the manufacturing process are outsourced.
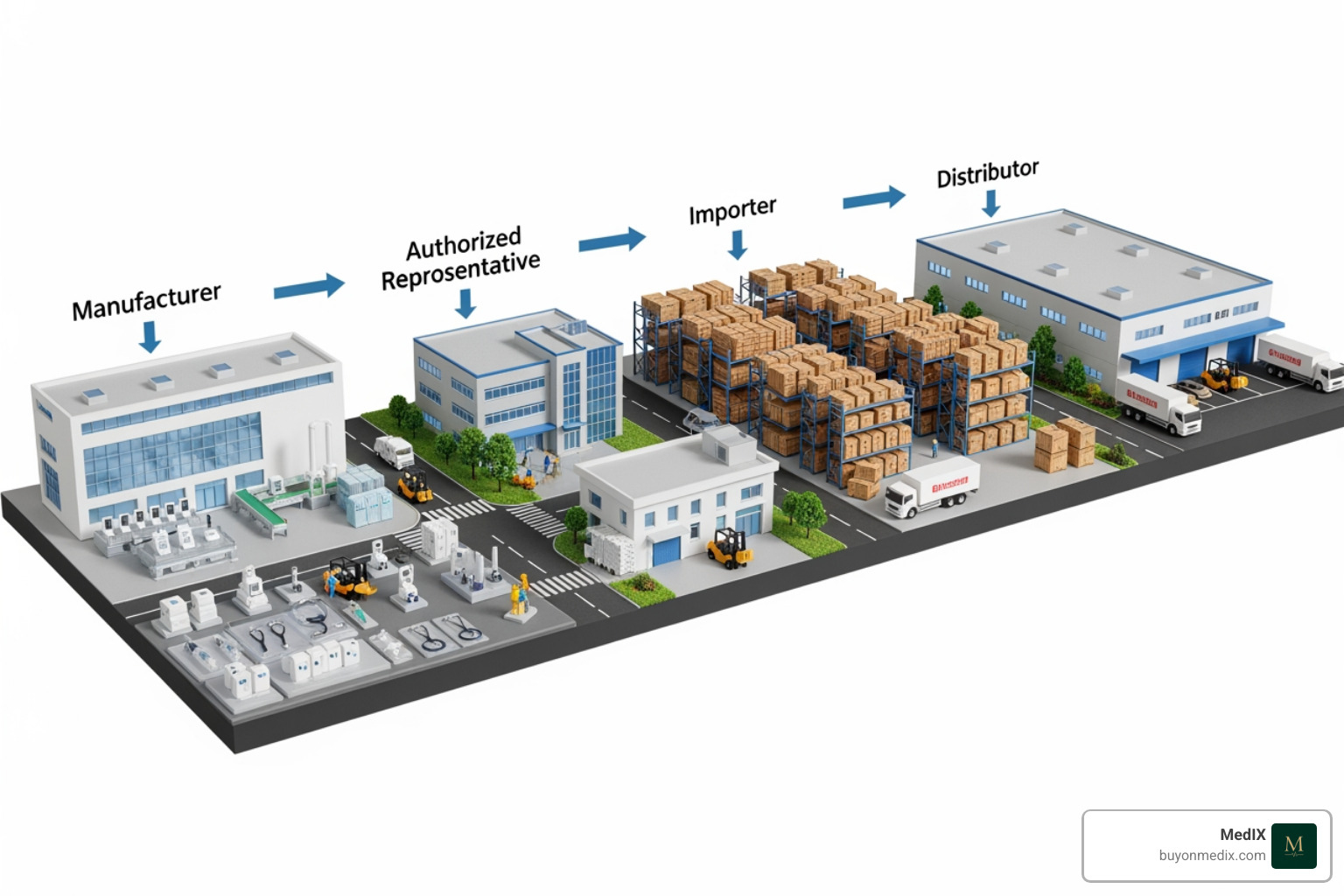
Key roles in the supply chain include:
- Manufacturer: The entity that manufactures a device (or has it designed and manufactured) and markets it under their own name. They initiate the CE marking process, conduct the conformity assessment, and issue the EU Declaration of Conformity.
- Importer: An EU-based entity that places a device from a non-EU country on the market. They must verify the manufacturer's compliance, including the presence of the CE mark and required documentation.
- Distributor: Anyone in the supply chain, other than the manufacturer or importer, who makes a device available. They must verify that the device bears the CE mark and is accompanied by the necessary information.
If an importer or distributor markets a product under their own name, they assume the full responsibilities of a manufacturer. Likewise, if a third party significantly modifies a device, they may become the new manufacturer and need to start a new CE marking process.
Key Responsibilities for Manufacturers of CE marked devices
Manufacturers must fulfill several critical obligations to ensure the safety and performance of their devices:
- Meet General Safety and Performance Requirements (GSPRs): The device must comply with the comprehensive GSPRs listed in Annex I of the MDR, covering everything from design and materials to risk management.
- Prepare and Maintain Technical Documentation: A detailed "technical file" must be created and maintained as proof of conformity. It includes design information, risk analysis, clinical data, and more, and must be available for inspection for at least 10 years (15 for implantable devices).
- Conduct a Clinical Evaluation: A thorough clinical evaluation must be performed and documented in a Clinical Evaluation Report (CER) to confirm the device's safety and performance.
- Implement a Risk Management System (RMS): A continuous RMS, often based on the EN ISO 14971 standard, must be established to identify, evaluate, and control risks throughout the device's lifecycle.
- Implement Post-Market Surveillance (PMS): Manufacturers must proactively collect and review data from devices in use. This includes Post-Market Clinical Follow-up (PMCF) for higher-risk devices to confirm long-term safety and performance.
- Register in EUDAMED and Assign a UDI: Devices and manufacturers must be registered in the European Database for Medical Devices (EUDAMED). Each device also requires a Unique Device Identification (UDI) for improved traceability.
- Engage a Notified Body: For most devices (Class IIa, IIb, and III), an independent Notified Body must be engaged to audit the Quality Management System and review technical documentation.
- Appoint a Person Responsible for Regulatory Compliance (PRRC): A designated individual with expertise in medical devices must be appointed to oversee regulatory compliance.
Navigating the EU Medical Device Regulation (MDR)
The regulatory landscape for CE marked devices changed significantly with the Medical Device Regulation (EU) 2017/745 (MDR), which replaced the previous directives. The MDR, applicable since May 26, 2021, established a more robust, transparent, and sustainable regulatory framework, emphasizing a lifecycle approach to device safety.

Understanding the Classification of CE marked devices
A foundational step is correctly classifying your device according to the MDR's risk-based system. Classification dictates the required conformity assessment route and the level of Notified Body involvement. The system is based on factors like the device's intended purpose, invasiveness, and duration of use.
Here’s a simplified overview of the medical device classes under the MDR:
| Device Class | Risk Level | Examples | Notified Body Involvement |
|---|---|---|---|
| Class I | Low | Non-sterile instruments, examination gloves, hospital beds. | Generally, self-declaration. NB required if sterile (Is), has a measuring function (Im), or is a reusable surgical instrument (Ir). |
| Class IIa | Medium | Hearing aids, contact lenses, surgical drapes. | Yes (QMS audit, technical documentation review). |
| Class IIb | Medium/High | Infusion pumps, ventilators, blood bags, bone plates. | Yes (Stricter QMS audit, design dossier review). |
| Class III | High | Vascular implants, replacement heart valves, hip implants. | Yes (Most stringent audits, full design dossier review, expert panel consultation). |
Note: In-Vitro Diagnostic Medical Devices (IVDs) are classified separately under the IVDR into Classes A, B, C, and D.
Misclassifying a device can lead to significant compliance issues, so careful review of the rules in Annex VIII of the MDR is critical.
Core EU MDR Requirements for Compliance
The MDR introduced several key requirements to improve device safety and transparency:
- Improved Clinical Evidence: Manufacturers must provide more robust clinical evidence to demonstrate safety and performance, often requiring new or updated clinical investigations.
- Stricter Post-Market Surveillance (PMS): The MDR mandates a proactive, systematic approach to collecting and analyzing data from devices on the market, including active Post-Market Clinical Follow-up (PMCF) for higher-risk devices.
- Person Responsible for Regulatory Compliance (PRRC): Manufacturers must appoint a PRRC with expert knowledge to oversee regulatory compliance activities.
- Supply Chain Transparency and Traceability: The Unique Device Identification (UDI) system and the EUDAMED database improve traceability and transparency, allowing for rapid response to safety issues.
- Lifecycle Approach: Compliance is an ongoing process, requiring continuous monitoring of a device's safety and performance from conception to disposal.
For a detailed look at the legal text, consult a copy of the Medical Devices Regulation (MDR).
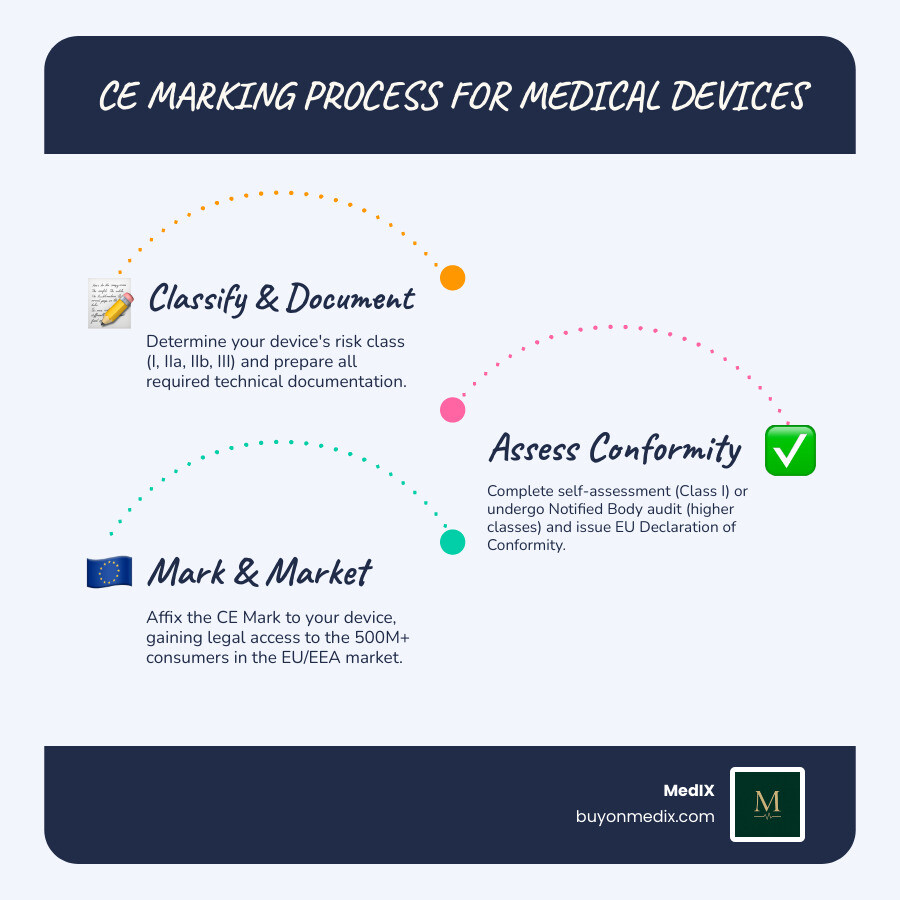
The Step-by-Step Process for CE Marked Devices
Obtaining CE marking for a medical device follows a structured process, though the exact path depends on the device's classification. The core principles, however, remain consistent.
The general steps are:
- Determine Applicable Legislation and Classify Your Device
- Fulfill Requirements and Prepare Technical Documentation
- Implement a Quality Management System (QMS)
- Undergo Conformity Assessment
- Draft the EU Declaration of Conformity
- Affix the CE Mark
Step 1: Determine Applicable Legislation and Classify Your Device
First, identify all relevant EU legislation, which for most medical devices is the Medical Devices Regulation (EU) 2017/745 (MDR). Next, correctly classify your device using the risk-based rules in Annex VIII of the MDR. This classification (Class I, IIa, IIb, or III) is critical as it dictates the entire conformity assessment path. For "borderline products" where the classification is unclear, consulting a competent authority is advisable.
Step 2: Fulfill Requirements and Prepare Technical Documentation
You must ensure your device meets all General Safety and Performance Requirements (GSPRs) from Annex I of the MDR. This is demonstrated through comprehensive technical documentation (also known as a technical file). This file is the core evidence of conformity and includes device descriptions, design and manufacturing information, risk analysis (per EN ISO 14971), clinical evaluation data (CER), labeling, and test results. This documentation must be kept for at least 10 years (15 for implantables). A robust Quality Management System (QMS), often certified to ISO 13485, must also be implemented.
Step 3: Undergo Conformity Assessment and Affix the CE Mark
The conformity assessment procedure is determined by the device class:
- Class I Devices (lowest risk): Manufacturers can typically perform a self-declaration of conformity. This involves preparing the technical documentation and signing the EU Declaration of Conformity without mandatory Notified Body involvement (unless the device is sterile, has a measuring function, or is a reusable surgical instrument).
- Class IIa, IIb, and III Devices: These higher-risk devices require the involvement of a Notified Body. The NB will audit the manufacturer's QMS and review the technical documentation. For the highest-risk devices, this includes a full design dossier review.
Upon successful assessment, the Notified Body issues a CE certificate. The manufacturer then drafts and signs the EU Declaration of Conformity and can affix the CE mark to the device. The mark must be visible, legible, and accompanied by the Notified Body's four-digit ID number if one was involved.
Key Players in the CE Marking Ecosystem
The CE marking process involves several key players beyond the manufacturer who ensure the safety and compliance of CE marked devices. Understanding their roles is vital for navigating the regulatory landscape.
The Role of a Notified Body
A Notified Body (NB) is an independent organization designated by an EU Member State to assess the conformity of medium- and high-risk medical devices. They are a mandatory partner for all devices except the lowest-risk Class I products.
Key functions of a Notified Body include:
- Independent Assessment: Conducting audits of a manufacturer's Quality Management System (QMS) and reviewing technical documentation to verify compliance with the MDR.
- Issuing CE Certificates: Issuing a CE certificate upon successful assessment, which is a prerequisite for the manufacturer to affix the CE mark.
- Ongoing Surveillance: Performing periodic surveillance audits to ensure the manufacturer maintains compliance throughout the certificate's validity.
- Expert Panel Consultation: For certain high-risk devices, the NB must consult with expert panels before issuing a certificate.
Choosing the right NB is a critical strategic decision. You can search for notified bodies in the NANDO database to find an organization designated for your device type.
The Role of an EU Authorized Representative (EC REP)
Manufacturers located outside the EU must appoint an EU Authorized Representative (EC REP). This representative acts as the manufacturer's official point of contact within the EU, a critical link for CE marked devices.
The EC REP's responsibilities include:
- Regulatory Liaison: Serving as the primary contact for EU competent authorities and Notified Bodies, and responding to requests for information or documentation.
- Vigilance and Incident Reporting: Assisting the manufacturer with reporting serious incidents to the relevant national authorities.
- Documentation Access: Ensuring the EU Declaration of Conformity and technical documentation are available for inspection by authorities.
- Registration: Assisting with the registration of the manufacturer and devices in the EUDAMED database.
The EC REP ensures a non-EU manufacturer has a regulatory presence in the EU and can be held legally liable for defective devices in certain situations.
Frequently Asked Questions about CE Marking
Here are answers to some common questions about CE marked devices.
How long is a CE certificate valid for?
The CE mark itself does not expire, but the underlying CE certificate issued by a Notified Body does. Under the MDR, these certificates are typically valid for a maximum of five years. Before expiration, manufacturers must undergo a recertification process, which includes new audits and reviews.
The validity of a CE certificate can also be affected by:
- Significant changes to the device's design or intended purpose.
- Updates to regulations or harmonized standards.
- Failure to pass annual surveillance audits conducted by the Notified Body.
Maintaining compliance is an ongoing process that requires continuous updates to documentation and periodic renewal of certificates.
What is the difference between the CE mark and the "China Export" mark?
There is a persistent myth about a "China Export" mark that looks similar to the official CE mark but with the letters closer together. There is no official "China Export" mark. The European Commission has confirmed this is a misconception, often arising from incorrect reproduction of the official logo.
The official CE mark has specific proportions and spacing. A poorly reproduced mark on a medical device may indicate that the manufacturer has not followed the proper conformity assessment process. Always verify the mark's authenticity and ensure it is supported by a valid EU Declaration of Conformity.
Can a CE marked device be sold in the UK?
Yes, due to post-Brexit transitional arrangements, CE marked devices can continue to be placed on the market in Great Britain (England, Scotland, and Wales) until at least June 30, 2028.
Key points for UK market access:
- UKCA Mark: The UK has introduced its own UK Conformity Assessed (UKCA) mark, which will eventually become mandatory for market access in Great Britain.
- MHRA Registration: Even during the transition, manufacturers must register their CE marked devices with the UK's Medicines and Healthcare products Regulatory Agency (MHRA).
- Northern Ireland: CE marking remains the requirement for devices placed on the Northern Ireland market due to the Northern Ireland Protocol.
While CE marking currently allows access to the UK market, manufacturers should prepare for the eventual full implementation of the UKCA marking system.
Conclusion: Ensuring Compliance and Building Trust
CE marking is a comprehensive regulatory framework essential for ensuring the safety and performance of medical devices in the European market. It represents a manufacturer's commitment to meeting the stringent requirements of the EU MDR, from initial design and classification to post-market surveillance. Adherence to this process is not just a legal necessity; it builds trust with healthcare providers and patients.
For procurement managers, prioritizing properly CE marked devices is a critical step in sourcing safe, effective, and compliant medical technology. It is your assurance that the equipment has undergone rigorous scrutiny and meets legally binding European standards.
At MedIX, we simplify this process. Our global B2B marketplace connects hospitals and clinics with verified suppliers, ensuring the equipment you procure is fully compliant. We invite you to explore our platform to find certified and compliant CE marked devices from trusted global suppliers and equip your facility with confidence.
Expert voices
Insights from leaders transforming medical equipment procurement






More from our blog
Discover the latest trends in medical technology and procurement
Stay ahead of medical technology
Get the latest insights, research, and market updates delivered straight to your inbox
Ready to transform your procurement
Discover how MedIX can streamline your medical equipment sourcing process



