Transforming medical equipment procurement globally
Why Medical Device Classification Matters for Safe, Compliant Procurement
Classification of medical devices pdf resources are essential tools for understanding how regulatory authorities categorize medical equipment based on risk. Here's what you need to know:
Quick Answer:
- Class I (Low Risk): Simple devices like bandages and tongue depressors - subject to general controls only
- Class II (Moderate Risk): Devices like infusion pumps and surgical masks - require general controls plus special controls and typically a 510(k) clearance
- Class III (High Risk): Life-supporting devices like pacemakers and heart valves - require premarket approval (PMA) with clinical data
The classification system determines which regulatory pathway a device must follow before it can be legally marketed in the US or EU.
Understanding medical device classification is foundational to patient safety and market access. For procurement, a device's class (I, II, or III) directly impacts your timeline, compliance requirements, and budget.
The US system, from the Medical Device Amendments of 1976, is a risk-based framework. The FDA has classified roughly 1,700 different generic types of devices into 16 medical specialties to match regulatory control with the risk a device poses to patients and users.
Classification depends on a device's intended use (what it's designed to do) and its indications for use (the specific conditions it treats). A surgical scalpel and an implantable defibrillator, for example, carry vastly different risks and face different regulatory requirements.
For procurement managers, this matters because classification determines everything from the documentation you'll need to verify, to the lead time before a device reaches your facility, to the price you'll ultimately pay. On MedIX, we surface key regulatory information alongside supplier credentials so hospitals and clinics can quickly confirm that a device's class, approvals, and documentation match their internal compliance policies.
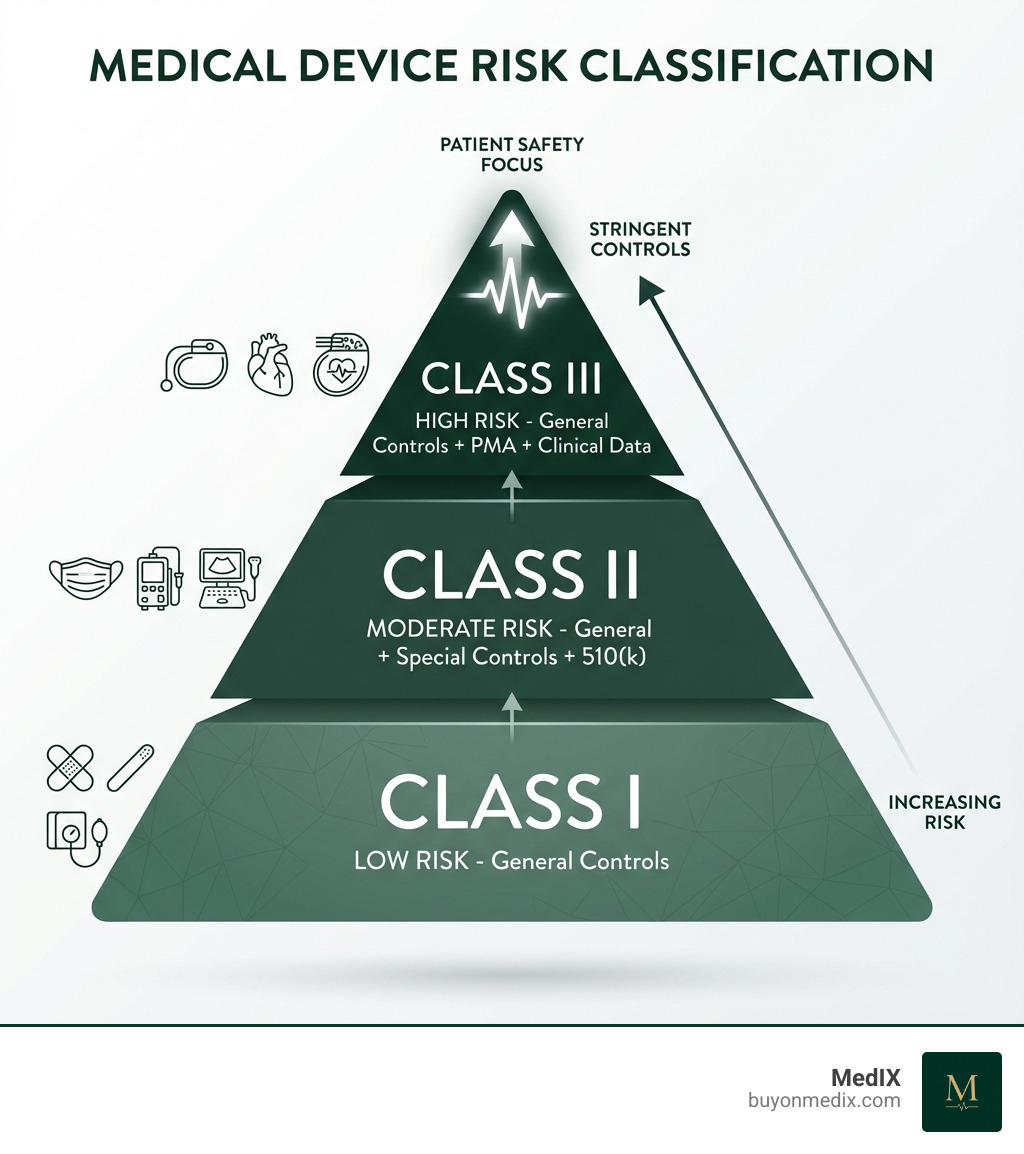
The US FDA Framework: Risk, Controls, and Pathways
The FDA's classification system, shaped by the 1976 Medical Device Amendments, ensures regulatory scrutiny is proportional to the risk a device poses to patients. Simply put, higher risk means more stringent controls.
The FDA's system categorizes devices into three primary classes: Class I, Class II, and Class III. This risk-based approach is codified in federal law, specifically Section 513 of the Federal Food, Drug, and Cosmetic Act (FD&C Act). The FDA has carefully classified around 1,700 generic types of devices, organizing them into 16 medical specialties, or panels.
The system's history includes 'pre-amendment' devices (marketed before 1976), 'post-amendment' devices (introduced after May 28, 1976), and 'transitional' devices that were previously regulated as drugs. This historical context helps explain the evolution of the regulatory environment.
Understanding the FDA's Three-Tier System
Let's explore the specifics of each class to understand the key differences in terms of risk and regulatory requirements:
Class I Devices (Low Risk): These devices pose the lowest potential risk to patients and users. They are subject to the least amount of regulatory control. Think of everyday medical items that rarely cause harm.
- Examples: Adhesive bandages, I.V. stands, sunglasses, elastic bandages, tongue depressors.
- Regulatory Requirement: Primarily subject to General Controls. Most Class I devices (approximately 74%, or 572 types) are exempt from Premarket Notification (510(k)) requirements.
Class II Devices (Moderate Risk): These devices present a moderate risk to patients. General Controls alone are typically insufficient to assure their safety and effectiveness, so they require additional measures.
- Examples: Syringes, surgical masks, powered wheelchairs, clinical electronic thermometers, infusion pumps.
- Regulatory Requirement: Subject to General Controls and Special Controls. Most Class II devices require Premarket Notification (510(k)) clearance from the FDA.
Class III Devices (Highest Risk): These are the big leagues of medical devices, carrying the greatest potential risk. They are often life-sustaining, life-supporting, or implantable, and their failure could result in serious injury or death.
- Examples: Heart valves, implantable pacemakers, implantable neuromuscular stimulators.
- Regulatory Requirement: Subject to General Controls and Premarket Approval (PMA) from the FDA. This is the most stringent regulatory pathway, often requiring extensive clinical data.
General Controls vs. Special Controls
The FDA employs a layered approach to regulatory oversight, ensuring that every device, regardless of its risk class, meets fundamental safety criteria. This layering is achieved through what we call General Controls and Special Controls.
General Controls: These are the foundational regulatory requirements that apply to all medical devices, unless specifically exempted. They are authorized by various sections of the FD&C Act (e.g., 501, 502, 510, 516, 518, 519, and 520).
- Key components of General Controls include:
- Establishment Registration: Manufacturers and distributors must register their facilities with the FDA.
- Device Listing: All devices manufactured must be listed with the FDA.
- Quality System Regulation (QSR): Devices must be manufactured under a quality system (21 CFR Part 820) to ensure consistent quality and safety.
- Labeling Requirements: Devices must have appropriate labeling, including intended use, warnings, and instructions.
- Medical Device Reporting (MDR): Manufacturers must report adverse events associated with their devices.
- Premarket Notification (510(k)): While most Class I devices are exempt, this is a General Control that applies to many Class I and most Class II devices.
- Prohibition against adulterated or misbranded devices: Devices must meet purity and labeling standards.
- Key components of General Controls include:
Special Controls: These are additional, device-specific requirements that come into play for Class II devices when General Controls alone are insufficient to assure safety and effectiveness.
- Special Controls typically include:
- Performance Standards: Mandatory standards that a device must meet (e.g., electrical safety, biocompatibility).
- Postmarket Surveillance: Requirements for monitoring a device's performance after it has entered the market.
- Patient Registries: For certain devices, collecting data on patient outcomes over time.
- Special Labeling Requirements: Specific warnings, precautions, or instructions beyond general labeling.
- Guidelines and Recommendations: Non-binding guidance documents from the FDA to aid compliance.
- Special Controls typically include:
You can learn more about these essential regulatory controls directly from the FDA: General and Special Controls explained.
Key Regulatory Pathways: 510(k), PMA, and De Novo
Once a device's class is determined, the next crucial step is to identify its regulatory pathway to market. This largely dictates the type of premarket submission required and the amount of data needed to demonstrate safety and effectiveness.
Premarket Notification (510(k)):
- When Required: Most Class II devices, and some Class I devices not exempt from premarket review.
- Purpose: To demonstrate that the new device is "substantially equivalent" to a legally marketed predicate device. This means it has the same intended use and the same technological characteristics, or different technological characteristics that do not raise new questions of safety and effectiveness and are as safe and effective as the predicate.
- Process: Manufacturers submit a 510(k) outlining how their device is similar to an existing one that has already been cleared by the FDA. This typically involves performance testing, engineering analysis, and sometimes clinical data, depending on the device's complexity.
Premarket Approval (PMA):
- When Required: All Class III devices.
- Purpose: This is the most stringent regulatory pathway, required for devices that are life-sustaining, life-supporting, implantable, or present a potential unreasonable risk of illness or injury. There's usually insufficient information to assure safety and effectiveness through general or special controls alone.
- Process: A PMA application requires extensive scientific evidence, often including clinical data from human studies, to demonstrate the device's safety and effectiveness. The FDA conducts a thorough review of the manufacturing process, labeling, and clinical data.
- You can find detailed information on PMA requirements: Device Advice on PMA.
De Novo Classification Process:
- When Used: For novel devices of low-to-moderate risk for which no legally marketed predicate device exists. Without the De Novo pathway, such devices would automatically default to Class III, requiring a PMA.
- Purpose: The De Novo process allows the FDA to classify novel devices into Class I or Class II, based on their risk profile, establishing a new classification regulation and often a new product code. This is an expedited mechanism compared to a full PMA for devices that don't truly belong in Class III.
- Process: Manufacturers submit a De Novo request, providing data to demonstrate that the device is safe and effective and that general controls (and special controls, if applicable) are sufficient to mitigate any risks. After FDA review, if granted, the device is reclassified, and a new regulatory path is established for similar future devices.
These pathways are critical for ensuring that medical devices reach the market safely and effectively, providing confidence in the equipment we procure for our hospitals and clinics.
Global Perspectives: A Comparison of US and EU Systems
While the US FDA system is a cornerstone of medical device regulation, it's important to recognize that medical devices are a global commodity. Many manufacturers operate internationally, and regulatory frameworks can vary significantly between regions. This is where the concept of regulatory harmonization comes into play. Organizations like the Global Harmonization Task Force (GHTF) – and its successor, the International Medical Device Regulators Forum (IMDRF) – have worked tirelessly to align regulatory systems worldwide. Their goal is to simplify the process for manufacturers, reduce compliance costs, and ultimately speed up patient access to innovative technologies, all while upholding public health standards.
Despite these efforts, key differences in approach persist between major regulatory bodies like the US FDA and the European Union's Medical Device Regulation (MDR). Both systems are risk-based, but their classification rules, number of classes, and regulatory pathways have distinct characteristics. Understanding these differences is crucial for any global medical equipment procurement strategy.
The European Union (EU) MDR Classification
The European Union's medical device landscape underwent a significant change with the introduction of the Medical Device Regulation (MDR 2017/745). This regulation, like the FDA's system, employs a risk-based approach, but it uses a different set of rules and a more granular classification structure.
Four Main Classes: The EU MDR classifies medical devices into four main categories:
- Class I (Lowest Risk): Similar to US Class I, these are low-risk devices.
- Class IIa: Moderate risk, often involving transient or short-term contact with the body.
- Class IIb: Higher moderate risk, including many implantable devices or those with more significant interaction with the body.
- Class III (Highest Risk): Comparable to US Class III, these are high-risk devices, often life-sustaining or implantable.
Subclasses within Class I: The EU MDR further refines Class I with specific subclasses:
- Class Is: Devices supplied in sterile condition (e.g., sterile examination gloves).
- Class Im: Devices with a measuring function (e.g., non-invasive blood pressure monitors).
- Class Ir: Reusable surgical instruments (e.g., scalpels, forceps).
22 Classification Rules: Instead of broad descriptions, the EU MDR relies on a detailed, rule-based system outlined in Annex VIII of the regulation. There are 22 specific rules that guide the classification of medical devices based on criteria such as invasiveness, duration of contact, whether the device is active, and special considerations for certain device types (e.g., those incorporating medicinal substances or animal tissues). If multiple rules apply, the strictest rule leading to the highest classification takes precedence.
Notified Body Involvement: A significant differentiator from the US system is the pervasive role of 'Notified Bodies' in the EU. These are independent third-party organizations designated to assess the conformity of medical devices with the MDR. For Class Is/Im/Ir devices, a Notified Body is involved in specific aspects (sterility, measurement, reusability). For Class IIa, IIb, and III devices, Notified Body involvement is extensive, including review of the manufacturer's quality management system and technical documentation.
IVD Classification: For in-vitro diagnostic devices (IVDs), the EU also has a separate, 7-rule classification system under the IVDR (In Vitro Diagnostic Regulation 2017/746), ranging from Class A (lowest risk) to Class D (highest risk).
For a comprehensive understanding of the EU's classification rules, we often refer to detailed guidance documents such as this one: PDF guide on EU classification.
[TABLE] comparing US FDA vs. EU MDR Device Classification
To help us visualize the differences, here's a comparative overview of the US FDA and EU MDR classification systems:
| Feature | US FDA Classification System | EU MDR Classification System |
|---|---|---|
| Governing Body | Food and Drug Administration (FDA) | European Commission, Member State Competent Authorities, Notified Bodies |
| Number of Classes | 3 (Class I, Class II, Class III) | 4 (Class I, Class IIa, Class IIb, Class III) |
| Risk Level Comparison | Class I (Low), Class II (Moderate), Class III (High) | Class I (Low), Class IIa (Moderate), Class IIb (Higher Moderate), Class III (High) |
| Classification Basis | Risk-based, considering intended use, indications for use, and technological characteristics. Broad categories with specific examples and regulations. | Risk-based, rule-based system (22 rules in Annex VIII MDR) considering invasiveness, duration of contact, active status, and special device features. |
| Primary Regulatory Pathway for Moderate/High Risk | Premarket Notification (510(k)) for most Class II devices; Premarket Approval (PMA) for Class III devices. | Conformity Assessment by a Notified Body (required for Class Is, Im, Ir, IIa, IIb, III). CE Marking required. |
| Key Differentiator | Substantial Equivalence (510(k)); De Novo pathway for novel devices. | Rule-based classification; extensive Notified Body involvement for higher classes; specific subclasses for Class I (Is, Im, Ir). |
| IVD Classification | Integrated into the 3-class system. | Separate 7-rule system (Class A, B, C, D) under IVDR 2017/746. |
A Practical Guide to the Classification of Medical Devices PDF Resources
Navigating the complexities of medical device classification can feel like a labyrinth, but with the right tools and strategies, it becomes a manageable process. For manufacturers, correctly classifying a device is not just a regulatory hurdle; it's a strategic decision that profoundly impacts the entire product development and market access pathway. An incorrect classification can lead to significant delays, increased costs, and even regulatory non-compliance. Our aim at MedIX is to simplify compliant procurement, and that starts with knowing exactly what each device entails.
The implications of classification are far-reaching. It dictates the type of premarket submission, the extent of clinical data required, the quality system requirements, and even post-market surveillance obligations. All of these factors directly influence the time and cost to market, making accurate classification a critical first step.
How to Determine the classification of medical devices pdf for Your Product
For manufacturers in the US, the FDA provides invaluable resources to help determine a device's classification. Here's how we recommend approaching it:
Start with the Intended Use and Indications for Use: Before you even touch a database, clearly define what your device is designed to do (intended use) and for what specific medical condition or purpose it will be used (indications for use). These are the most critical factors the FDA considers.
Search the FDA Classification Database: This is your go-to resource. The FDA's Product Classification Database allows you to search for existing devices.
- By Device Name or Keyword: Start by searching for your device's generic name (e.g., "infusion pump," "surgical mask") or keywords that describe its function.
- By Predicate Device: If you know of a legally marketed device that is similar to yours (a potential predicate), search for that device. This can provide a direct path to its classification.
Identify the Regulation Number: Once you find a similar device, you'll see a 7-digit Regulation Number (e.g., 21 CFR 880.2920 for a Clinical Mercury Thermometer). This number references the specific section in the Code of Federal Regulations (CFR) that describes that generic type of device. The FDA has established approximately 1,700 such regulation numbers. You can view the full CFR text for any regulation number by using the CFR Search page.
Identify the Product Code: Each generic type of device also has a unique three-letter Product Code (e.g., FLL for a Clinical Electronic Thermometer). The FDA has approximately 6,500 product codes assigned across its 16 medical specialties (panels). A single regulation number can sometimes cover many different product codes, so choose the one that most precisely matches your device's characteristics.
Check for Exemptions: Some Class I and even certain Class II devices are exempt from Premarket Notification (510(k)) and/or parts of the Quality System Regulation. Always check the classification regulation for any listed exemptions. You can also explore the Medical Device Exemptions database. For example, a manual toothbrush is a Class I device with stated exemptions from general controls.
By systematically using these resources, manufacturers can effectively determine the classification of medical devices pdf for their products, laying the groundwork for a successful regulatory strategy.
Formal FDA Assistance: The 513(g) Request
Sometimes, even with the best detective work, a device's classification remains unclear, especially for novel technologies or borderline products. In such cases, manufacturers can seek formal guidance from the FDA through a 513(g) Request for Information.
When to Use It: A 513(g) request is appropriate when you need a formal determination from the FDA on whether your product is a medical device, its classification, or the type of premarket submission required. It's particularly useful for novel devices that don't clearly fit into existing categories or when you're uncertain about your device's intended use relative to existing predicates.
Purpose: This request is not a premarket submission itself, but rather a formal inquiry to clarify your device's regulatory status. It's a way to get a definitive answer directly from the agency.
Process: You submit a detailed description of your device, its intended use, indications for use, and any relevant scientific information. The FDA reviews this information and provides a written response.
- Review Cycle: The FDA aims for a 60-day review cycle for 513(g) requests.
- User Fees: There are user fees associated with 513(g) requests, with reduced fees available for eligible small businesses under the Small Business Determination (SBD) Program. The specific fee amounts vary by fiscal year and are updated annually by the FDA.
Getting Started: If you're unsure if your product even qualifies as a medical device, you can contact the CDRH Office of Compliance by email at DeviceDetermination@fda.hhs.gov. For submission instructions, refer to the guidance document "FDA and Industry Procedures for Section 513(g) Requests for Information": 513(g) Requests for Information.
Utilizing the 513(g) process can save manufacturers significant time and resources by providing regulatory clarity early in the development cycle, preventing costly missteps down the line.
Frequently Asked Questions about Medical Device Classification
We often encounter common questions about medical device classification. Let's address some of the most pressing ones to further explain this critical topic.
What is the primary purpose of classifying medical devices?
The primary purpose of classifying medical devices is to ensure the safety and effectiveness of devices sold in the U.S. by matching the level of regulatory control to the level of risk posed by a device. This risk-based system protects patients while facilitating market access for safe products. It means that a simple tongue depressor won't go through the same rigorous approval process as an artificial heart valve, allowing for efficient allocation of regulatory resources and faster access to low-risk innovations. It’s about balancing innovation with patient protection.
How do I find the classification of a device similar to mine?
Finding the classification of a device similar to yours is a fundamental step in determining your own regulatory pathway. We recommend using the FDA's Product Classification Database. Here's how:
- Search by Keyword: Enter the generic name of your device (e.g., "surgical mask," "powered wheelchair") or descriptive keywords.
- Search by Competitor Product Name: If you know a competitor's product that is similar in intended use and technology, you can sometimes find its classification by searching for its name.
- Identify Regulation Number and Product Code: Once you find a similar device, note its 7-digit regulation number and 3-letter product code. These will guide you to the specific regulatory requirements.
- Review the Regulation: Click on the regulation number to read the official description in the Code of Federal Regulations. This will often include the device's class, any special controls, and exemptions.
Even subtle differences in intended use or technological characteristics can lead to different classifications, so always evaluate carefully.
What is the difference between a 510(k) and a PMA?
This is a common point of confusion, but understanding the distinction is key to navigating the US regulatory system:
510(k) (Premarket Notification): This is the most common pathway for Class II devices and some Class I devices. The primary goal of a 510(k) submission is to demonstrate that your new device is "substantially equivalent" to a legally marketed predicate device. This means it has the same intended use and similar technological characteristics, or different characteristics that don't raise new safety or effectiveness concerns. It's essentially showing that your device is "as safe and effective" as something already on the market, without necessarily proving absolute safety and effectiveness from scratch.
PMA (Premarket Approval): This is the most stringent FDA review process, required for Class III devices. Unlike a 510(k), a PMA requires robust scientific evidence, often including extensive clinical data from human trials, to prove the device's safety and effectiveness. There's no predicate device to claim substantial equivalence to; instead, manufacturers must independently demonstrate the device's benefits outweigh its risks. This pathway is reserved for devices that pose the highest risk, such as implantable pacemakers or heart valves.
A 510(k) is a comparison to an existing device, while a PMA is a comprehensive, standalone demonstration of safety and effectiveness.
Conclusion: From Classification to Compliant Procurement
Understanding the classification of medical devices pdf resources is more than just a regulatory exercise; it's a fundamental aspect of ensuring patient safety, driving innovation, and facilitating market access for essential medical equipment. We've explored how the US FDA's risk-based system, with its three classes and distinct regulatory pathways (510(k), PMA, and De Novo), carefully categorizes devices based on their intended use, indications for use, and potential risk. We also touched upon the EU MDR's more granular, rule-based system, highlighting the global efforts towards harmonization while acknowledging regional differences.
For manufacturers, grasping these classification nuances from the outset is paramount. It shapes every decision, from product design and testing to regulatory strategy and market launch. An accurate classification guides the entire product development pathway, influencing timelines, costs, and the ultimate success of bringing life-changing technologies to healthcare providers.
For hospitals and clinics, this knowledge empowers procurement teams to make informed decisions, ensuring that the medical equipment they acquire is not only fit for purpose but also fully compliant with the highest safety and effectiveness standards. At MedIX, our mission is to connect certified medical equipment suppliers with hospitals and clinics worldwide, simplifying procurement through AI-matching, rigorous compliance checks, and reliable global logistics. We believe that by understanding the intricate world of medical device classification, we can collectively ensure verified equipment and transparent transactions, ultimately enhancing healthcare delivery for everyone.
Ready to find compliant and certified medical equipment for your facility? Find certified medical equipment on MedIX today.
Expert voices
Insights from leaders transforming medical equipment procurement






More from our blog
Discover the latest trends in medical technology and procurement
Stay ahead of medical technology
Get the latest insights, research, and market updates delivered straight to your inbox
Ready to transform your procurement
Discover how MedIX can streamline your medical equipment sourcing process



