Transforming medical equipment procurement globally

The Unique Challenges of Medical Device Logistics

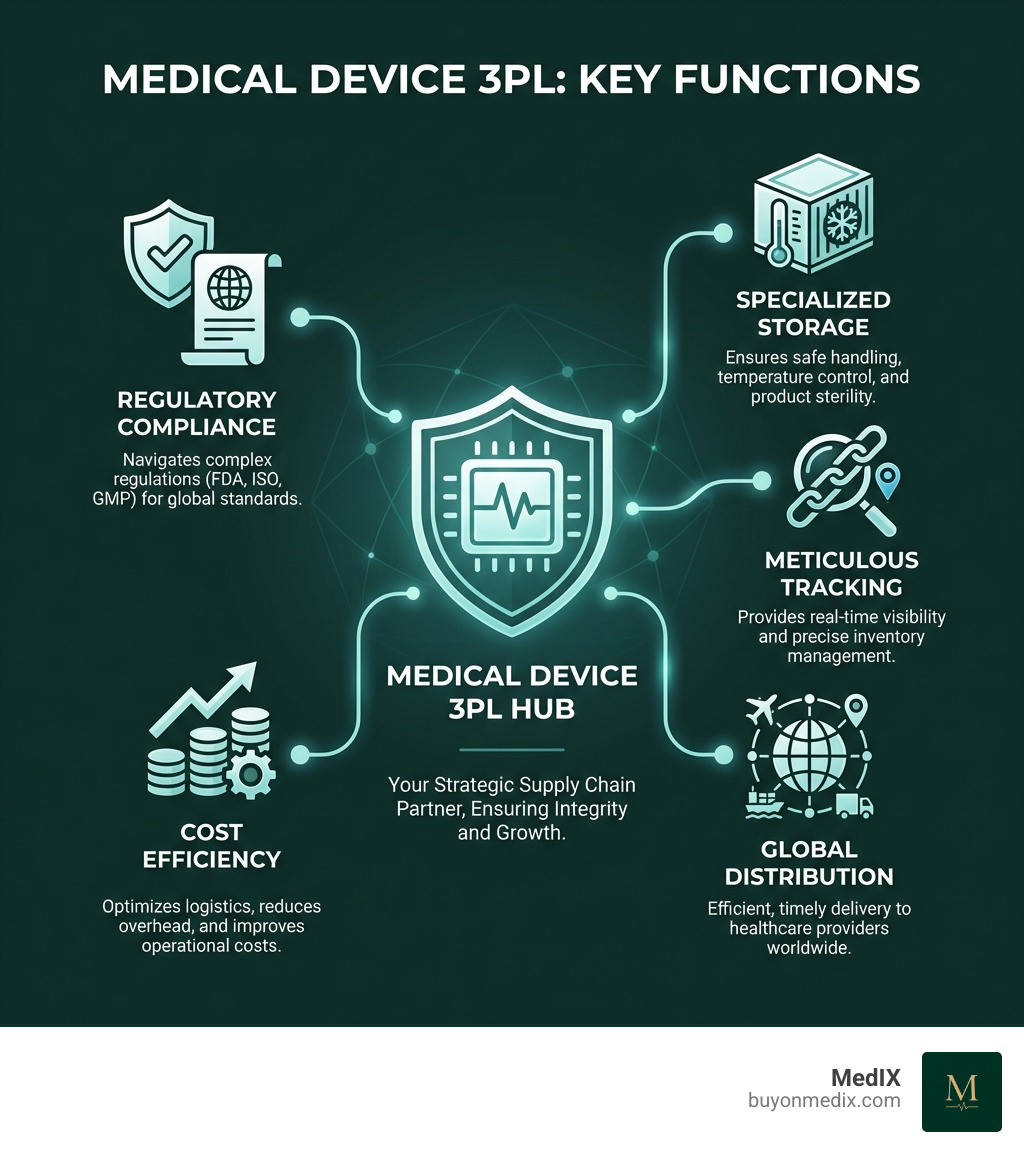
A medical device 3PL (Third-Party Logistics) is a specialized partner handling the storage, order fulfillment, and distribution for medical devices. They provide:
- Specialized Expertise: Steers complex regulations (FDA, ISO, GMP).
- Product Integrity: Ensures safe handling, temperature control, and sterility.
- Efficiency: Provides meticulous tracking, inventory management, and timely delivery.
- Strategic Growth: Allows companies to focus on innovation and expand markets.
Medical devices are vital, and their journey from factory to patient is critical. Unlike regular products, they face unique challenges. Invisible damage—a tiny misalignment or a loose sterile seal—can be as risky as a delayed shipment. The medical device supply chain demands precision, strict rules, and constant care. This is where a specialized medical device 3PL comes in, with 58% of shippers planning to increase their use of outsourced logistics. These experts manage the heavy lifting, allowing manufacturers to focus on innovation and R&D.
This guide explains how a medical device 3PL becomes an essential partner, ensuring your critical products arrive safely, on time, and fully compliant.
Managing logistics for medical devices is a world apart from consumer goods. The stakes are incredibly high, introducing unique challenges that demand specialized solutions.
First, product fragility is a major concern. Many devices contain delicate components susceptible to "invisible damage" from minor mishandling, which can cause functional failure or contamination. Second, maintaining sterility is paramount for surgical instruments and implantables, where any breach can have severe patient health consequences. Third, regulatory problems loom large. Non-compliance with strict global standards can lead to fines, recalls, and reputational damage. Finally, medical devices are often high-value inventory, making their safe and efficient movement crucial for business profitability and patient access. For a deeper dive, you can explore more on the healthcare supply chain.
Meticulous Tracking: Serialization and Lot Control
In medical devices, meticulous end-to-end tracking, including serialization and lot control, is crucial. A large portion of devices requires tracking via a Unique Device Identification (UDI) assigned to each product. This provides a complete product lifecycle history and unparalleled visibility, allowing a specific device to be traced from manufacturing to the patient.
This level of tracking is vital for several reasons:
- Recall Management: If a defect is found or a recall is necessary, serialization allows for rapid identification and removal of affected lots from the market.
- Preventing Counterfeits: A verifiable history for each device helps combat counterfeit medical products.
- Improved Patient Safety: Knowing a device's exact lineage helps ensure its authenticity and integrity.
- Regulatory Compliance: Many regulatory bodies mandate serialization, making it a non-negotiable aspect of logistics.
Without this granular tracking, managing risks and ensuring patient safety becomes significantly more challenging.
Shelf-Life and Environmental Sensitivities
Many medical products have a limited shelf life, complicating their logistics. This necessitates strict adherence to principles like First-In-First-Out (FIFO) and, ideally, First-Expired-First-Out (FEFO). With FEFO, products closest to their expiration date are shipped first, minimizing waste and ensuring patients receive effective products. High inventory accuracy is mission-critical for medical device order fulfillment.
Beyond expiration dates, many devices are sensitive to environmental conditions:
- Temperature Control: Many devices and biologics require precise temperature ranges, from ambient to cryogenic (-80°C), to maintain efficacy.
- Humidity Monitoring: Excessive humidity can cause corrosion or damage to electronic components.
- Light Sensitivity: Some products can be damaged by light, requiring specialized opaque packaging.
- Contamination Risk: The integrity of a device can be compromised by dust, microbes, or chemicals. As one source notes, "Contamination isn’t a risk; it’s a deal-breaker."
A medical device 3PL must have the infrastructure to manage these sensitivities, ensuring products remain in optimal condition.
Handling Diverse and Sensitive Medical Equipment
The variety of medical devices means a "one-size-fits-all" logistics approach is ineffective. Each category demands specific handling and storage.
Here’s a glimpse at the diversity:
- Diagnostic Imaging Devices: These contain sensitive magnets that can be affected by nearby electromagnetic activity. They must be stored in magnetically safe zones.
- Surgical Instruments: Sterility is non-negotiable. Their sterile packaging must remain sealed during transit and storage.
- Implantable Devices: Pacemakers or joint replacements require trained precision in handling to protect their integrity and sterility.
- Monitoring Devices: Featuring delicate displays and sensors, these devices need careful handling to avoid damage from pressure.
- Laboratory Equipment: Items like microscopes and centrifuges are fragile and sensitive to shock, requiring specialized packaging and handling.
This list shows why a medical device 3PL moves with purpose, understanding they are shipping tools that power surgeries or monitor a patient's heartbeat. For more information on equipment types, refer to our insights on Medical Equipment.
How a Specialized Medical Device 3PL Solves These Challenges
Partnering with a specialized medical device 3PL is a strategic necessity. These partners are built to address the stringent demands of the healthcare sector, offering expertise, infrastructure, and technology that general providers cannot match.

A dedicated medical device 3PL provides:
- Regulatory Expertise: Specialists who live and breathe healthcare regulations.
- Purpose-Built Facilities: Warehouses with temperature-controlled zones, secure storage, and cleanroom capabilities.
- Advanced Technology: Cutting-edge systems for tracking, inventory management, and data analysis.
- Trained Personnel: Team members trained in medical device handling, quality control, and compliance.
- Quality Management Systems (QMS): Rigorous QMS frameworks ensuring consistent quality and adherence to standards.
This comprehensive approach mitigates risks and streamlines operations, ensuring devices reach their destination safely and compliantly.
Ensuring Regulatory and Quality Compliance
For medical devices, compliance is foundational. A specialized medical device 3PL acts as your frontline defense against regulatory pitfalls.
Our partners steer complex frameworks such as:
- FDA 21 CFR Part 820: The quality system regulation for medical devices in the United States.
- ISO 13485 Certification: An international standard for a quality management system for medical devices.
- Good Manufacturing Practices (GMP): Principles ensuring products are consistently produced and controlled to quality standards.
- Good Distribution Practices (GDP): A quality system for warehouses and distribution centers, ensuring product integrity.
A specialized medical device 3PL manages all aspects of regulatory compliance, from holding licenses to implementing rigorous checks. This reduces compliance risks and the potential for costly recalls. They stay updated on evolving regulations, ensuring your logistics are always audit-ready and compliant with the latest import/export requirements.
Specialized Services and Infrastructure
The unique needs of medical devices demand specialized services and infrastructure. A medical device 3PL invests heavily in these capabilities to protect product integrity.
Consider these specialized offerings:
- Temperature-Controlled Storage: Modules are available from ambient to -80°C for thermosensitive products, with precise monitoring of temperature and humidity.
- Automated Storage and Retrieval Systems (AS/RS): These systems enable efficient storage, minimize human contact, optimize space, and improve inventory accuracy.
- Secure Warehousing: High-value devices require improved security, including restricted access, surveillance, and robust inventory control.
- White-Glove Delivery: For sensitive equipment, this service includes specialized handling, installation, and even technical support at the delivery site.
- Reverse Logistics (Returns & Recalls): Managing returns and recalls requires a structured, compliant process for inspection, disposition, or destruction while maintaining traceability.
- Kitting and Assembly: A 3PL can perform value-added services like assembling surgical kits in a controlled environment, reducing complexity for manufacturers.
This infrastructure ensures every medical device is handled with the precision and care it demands.
Smart Order Fulfillment and Technology
Smart order fulfillment integrates technology with meticulous workflows to achieve accuracy, speed, and transparency. A medical device 3PL leverages cutting-edge solutions to optimize every step.
Key components include:
- Robust Warehouse Management Systems (WMS): The backbone of operations, a WMS manages inventory, directs picking and packing, and handles shelf life (FEFO), lot numbers, and serial numbers.
- Real-Time Inventory Visibility: Advanced scanning (barcode, RFID) and integrated systems provide critical knowledge of inventory location and status.
- Real-Time GPS Shipment Tracking: Shipments are fully traceable via GPS from dispatch to delivery, offering peace of mind and proactive management.
- Data Analytics: Powerful tools provide insights into supply chain performance, identify bottlenecks, and optimize routes.
- Process Validation: Every process is rigorously validated to ensure compliance and consistent quality.
- Quality Inspections: Devices undergo thorough quality inspections before shipping to confirm packaging integrity and labeling accuracy.
This technological prowess ensures the right device, in perfect condition, reaches its destination precisely when needed.
The Strategic Business Benefits of Partnering with a 3PL
Partnering with a specialized medical device 3PL offers significant strategic advantages that optimize resources, expand reach, and strengthen your market position.
The key benefits include:
- Cost Reduction: Leveraging economies of scale and shared resources leads to operational savings.
- Scalability: Easily adjust logistics operations to meet fluctuating demand without heavy capital investment.
- Risk Mitigation: Expert management of compliance, product integrity, and supply chain disruptions reduces risks.
- Market Expansion: Access to established global networks facilitates entry into new markets.
- Improved Customer Experience: Faster, more accurate, and damage-free deliveries improve satisfaction.
A medical device 3PL is a strategic partner that empowers your business to thrive in a highly competitive and regulated environment.
Focusing on Core Competencies
A compelling reason to partner with a medical device 3PL is the ability to focus on your core competency: innovation. Developing groundbreaking medical devices is a resource-intensive endeavor requiring immense focus on research and development (R&D).
By outsourcing logistics, companies can:
- Accelerate R&D: Free up internal resources to invest more heavily in product development and clinical trials.
- Improve Product Innovation: Allow engineers and scientists to concentrate on designing the next generation of devices.
- Improve Speed to Market: A specialized 3PL can streamline the journey from manufacturing to market, getting new products to patients faster.
- Reduce Operational Burden: Offloading logistics allows leadership to concentrate on strategic growth and core business objectives.
A medical device 3PL allows manufacturers to prioritize innovation, knowing that the intricate logistics are in expert hands.
Cost-Effectiveness and Scalability
The financial and operational benefits of partnering with a medical device 3PL are substantial. Building an in-house logistics infrastructure requires significant capital and ongoing costs.
A 3PL offers a more agile and cost-effective model:
- Economies of Scale: 3PLs negotiate better rates with carriers and optimize labor, passing savings on to their partners.
- Shared Warehousing Costs: You benefit from shared, fully-equipped facilities without the large upfront capital investment of owning a warehouse.
- Reduced Capital Expenditure: Avoid the costs of acquiring land, constructing facilities, and purchasing equipment.
- Flexible Space and Labor: A 3PL can easily scale operations up or down based on demand, providing invaluable flexibility.
- Volume Shipping Discounts: High shipping volumes allow 3PLs to secure advantageous shipping rates that individual companies often cannot.
This flexibility and cost-efficiency enable medical device companies to grow and adapt without the prohibitive burdens of managing logistics internally.
The Role of a Medical Device 3PL in Global Expansion
For companies with global ambitions, a medical device 3PL is an indispensable partner for navigating the logistical and regulatory challenges of new international markets.
Our partners facilitate global distribution by:
- Navigating International Regulations: A global 3PL has the local expertise to ensure compliant operations across different countries' unique import/export requirements.
- Access to Global Distribution Networks: 3PLs possess extensive networks of warehouses and transportation partners, allowing companies to leverage existing infrastructure to expand rapidly.
- Compliant Customs Clearance: 3PLs manage complex customs documentation, duties, and taxes, ensuring smooth cross-border movement of goods.
- Acting as an Extension of Your Brand: A reputable medical device 3PL handles devices with care and professionalism, acting as a positive extension of your brand and strengthening customer relationships worldwide.
By leveraging a 3PL's global reach, companies can focus on market entry strategies, confident that their supply chain is robust and compliant. To learn more about connecting with verified partners, read about Global Medical Device Distributors.
Frequently Asked Questions about Medical Device 3PLs
Here are answers to some of the most common questions about how a medical device 3PL operates and their capabilities.
How does a 3PL ensure the sterility of medical devices?
Ensuring the sterility of sensitive medical devices is a top priority for a specialized medical device 3PL and is critical for patient safety and compliance.
Here's how our partners ensure sterility:
- Sealed Packaging Integrity: 3PLs implement strict protocols to ensure a device's primary sterile packaging remains sealed and undamaged throughout the supply chain.
- Controlled Environments: Many facilities feature controlled environments, like cleanrooms, with filtered air and controlled humidity to minimize contaminants.
- Contamination Prevention Protocols: Rigorous cleaning schedules, pest control, and strict personnel hygiene are standard operating procedures.
- Specialized Handling Procedures: Personnel are trained to handle sterile devices, minimizing contact and avoiding actions that could compromise the product's condition.
- Documented Quality Checks: Regular quality checks are performed and documented at receiving, during storage, and before shipment to inspect packaging for any damage.
These combined measures create a robust system to maintain device sterility and integrity.
What technology is crucial for a medical device 3PL?
The backbone of a high-performing medical device 3PL is its integrated, validated technology stack that supports precision, compliance, and visibility.
Crucial technologies include:
- A Robust Warehouse Management System (WMS): This central system manages inventory by lot, serial number, and expiration date (FEFO/FIFO) and directs warehouse activities.
- Real-Time Tracking and Visibility Platforms: These systems provide end-to-end transparency on inventory status, order progress, and live shipment location via GPS.
- Environmental Monitoring Sensors: IoT-enabled sensors continuously monitor and record conditions like temperature and humidity in storage and transit.
- Barcode and RFID Scanning for Serialization: These are critical for accurate inventory management and meticulous end-to-end tracking of individual devices.
- Validated Software Systems: All software must be validated to ensure it performs as intended and complies with regulations (e.g., FDA 21 CFR Part 11).
- Automation Technologies: AS/RS, robotics, and smart glasses improve efficiency, accuracy, and reduce human error.
This technology enables a medical device 3PL to operate with the precision demanded by the healthcare industry.
How does a 3PL help with product recalls?
A product recall is a critical event, and a specialized medical device 3PL can help manage the process with speed and precision.
Here's how a 3PL assists with product recalls:
- Rapid Identification of Affected Lots: Meticulous serialization and lot tracking allow a 3PL to swiftly pinpoint the exact affected units and their locations.
- Quarantine Procedures: The 3PL immediately implements strict quarantine procedures, isolating all affected products to prevent further distribution.
- Efficient Reverse Logistics: They manage the entire process of retrieving recalled products from the field, including coordinating transportation and providing return instructions.
- Detailed Tracking Records for Reporting: Every step of the recall is carefully documented, providing essential records for regulatory reporting and demonstrating compliance.
- Communication Support: A 3PL provides critical data and logistical support to facilitate the manufacturer's timely and accurate communication with regulators and customers.
By having these systems in place, a medical device 3PL significantly mitigates the risks associated with recalls, protecting patient safety and the manufacturer's brand.
Conclusion
The journey of a medical device is fraught with unique challenges, from product fragility and sterility to stringent regulations and meticulous tracking. For medical device companies, navigating this complexity while focusing on innovation is a difficult balancing act.
A specialized medical device 3PL is an indispensable ally. They offer a strategic partnership built on expertise, purpose-built infrastructure, and cutting-edge technology. Our partners ensure regulatory compliance, safeguard product integrity, and optimize the entire supply chain.
By entrusting your logistics to a specialized 3PL, you gain:
- Unwavering Compliance: Steer the web of FDA, ISO, GMP, and GDP regulations with confidence.
- Improved Safety: Ensure devices maintain sterility and integrity through specialized handling and controls.
- Operational Efficiency: Benefit from meticulous tracking, automated systems, and optimized processes.
- Strategic Focus: Reallocate resources to core competencies like R&D, accelerating innovation and growth.
In an industry where every shipment can impact a life, partnering with the right medical device 3PL future-proofs your supply chain. It ensures your critical products reach those who need them most, safely and without compromise.
At MedIX, we understand the critical nature of this industry. Our platform simplifies procurement through AI-matching, rigorous compliance checks, and reliable global logistics, ensuring verified equipment and transparent transactions worldwide. We connect you with certified medical equipment suppliers and the specialized partners who make their journey possible.
To learn more about optimizing your logistics strategy and connecting with trusted partners, explore our insights on Medical Device Logistics.
Expert voices
Insights from leaders transforming medical equipment procurement






More from our blog
Discover the latest trends in medical technology and procurement
Stay ahead of medical technology
Get the latest insights, research, and market updates delivered straight to your inbox
Ready to transform your procurement
Discover how MedIX can streamline your medical equipment sourcing process




