Transforming medical equipment procurement globally
Your Passport to European Markets: Understanding CE Certified Medical Devices
For procurement managers sourcing medical equipment for global healthcare institutions, understanding European compliance is key. A CE certified medical device is your ticket to one of the world's largest and most regulated markets.
CE stands for "Conformité Européenne," or "European Conformity." This mark is the manufacturer's declaration that their product complies with all European health, safety, performance, and environmental standards. For medical devices, CE marking is a non-negotiable requirement to sell within the European Economic Area (EEA), ensuring the free movement of goods across 32 countries. It tells regulators, providers, and patients that a device is safe and performs as intended.
This guide will walk you through the essentials of CE certification for medical devices, from regulations to compliance steps.
What is a CE Certified Medical Device and Why is it Significant?
A CE certified medical device is a product that the manufacturer declares meets all legal requirements for sale within the EEA. It is a mandatory requirement for any manufacturer wishing to place medical or in vitro diagnostic (IVD) devices on the EEA market. The significance of the CE mark is a profound commitment to patient safety and product performance. Here’s why it matters:
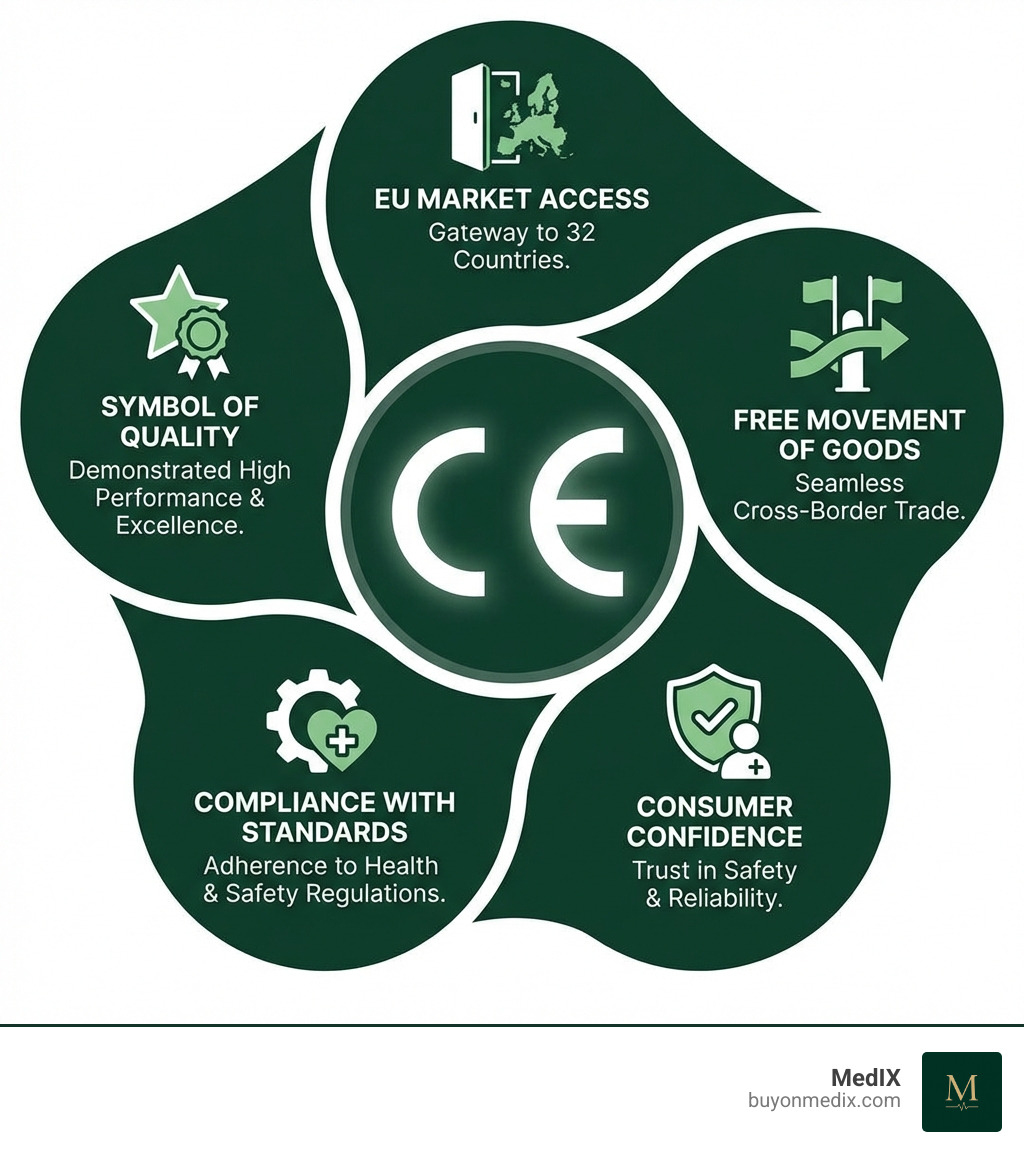
- Market Access: CE marking grants legal access to the 32 countries of the EEA, a market of nearly 500 million consumers. It allows for the free movement and sale of products, regardless of their origin.
- Symbol of Quality and Trust: The CE mark is recognized globally as a symbol of compliance with stringent European standards. It instills confidence in healthcare professionals and patients that the device is safe and performs as intended.
- Ensuring Patient Safety: The CE marking process is built around device safety and performance. It requires rigorous testing, risk management, clinical evaluation, and post-market surveillance to minimize risks.
- Manufacturer's Responsibility: By affixing the CE mark, the manufacturer takes full legal responsibility for the product's compliance throughout its lifecycle. This commitment is a cornerstone of the EU's regulatory framework.
In short, a CE certified medical device demonstrates a manufacturer's dedication to quality and safety, opening doors to the European market and building user trust.
The Regulatory Foundation: Understanding MDR and IVDR
The EU's regulatory landscape for medical devices is defined by two key regulations that replaced older directives: the EU Medical Device Regulation (MDR) and the In Vitro Diagnostic Regulation (IVDR).

EU Medical Device Regulation (MDR) - Regulation (EU) 2017/745
The MDR (Regulation (EU) 2017/745) took full effect on May 26, 2021, replacing the previous Medical Device Directive (MDD). It established a more robust, transparent, and sustainable regulatory framework focused on patient safety.
Key changes under the MDR include:
- Expanded Scope: The definition of a medical device now includes certain aesthetic products without a medical purpose (e.g., dermal fillers).
- Stricter Requirements: The General Safety and Performance Requirements (GSPRs) are more detailed than the previous "Essential Requirements," covering risk management, clinical evaluation, and post-market surveillance.
- Increased Scrutiny: High-risk devices (Class III and some Class IIb) face more stringent pre-market assessment.
- Lifecycle Approach: The MDR mandates a comprehensive approach to regulation, from design to post-market surveillance.
- Stronger Clinical Evidence: A more rigorous approach to clinical evaluation is required, with greater emphasis on device-specific data.
In Vitro Diagnostic Regulation (IVDR) - Regulation (EU) 2017/746
The IVDR (Regulation (EU) 2017/746) became fully applicable on May 26, 2022, replacing the previous IVD Directive. It mirrors the MDR's goals of improving safety and reliability for in vitro diagnostic products.
Notable changes under the IVDR include:
- New Risk-Based Classification: A new system (Classes A, B, C, D) aligns with global standards and moves more IVDs into higher-risk classes that require Notified Body oversight.
- Improved Performance Evaluation: IVDR requires a robust performance evaluation, covering scientific validity, analytical performance, and clinical performance.
- Greater Notified Body Involvement: A much larger percentage of IVDs now require assessment by a Notified Body, a shift from the previous self-declaration model.
Both regulations reinforce the EU's commitment to patient safety through greater transparency and traceability. Staying current with these More on the latest regulations is crucial for all industry stakeholders.
The Step-by-Step Process to Obtain a CE Certified Medical Device
Obtaining CE marking is a structured process with clear steps. The manufacturer is ultimately responsible for ensuring compliance and affixing the CE mark, though importers and distributors also have roles in maintaining compliance.
Let's break down the conformity assessment process.
Step 1: Classify Your Device
The first step is to accurately classify your medical device or IVD based on risk. This classification determines the required conformity assessment route.
- MDR Classes (Medical Devices): Class I (low risk), Class IIa (medium risk), Class IIb (medium-high risk), and Class III (high risk).
- IVDR Classes (In Vitro Diagnostics): Class A (low risk), Class B (medium risk), Class C (high risk), and Class D (highest risk).
Misclassification can lead to significant delays or non-compliance. Our Classification of Medical Devices PDF can provide more detailed guidance.
Step 2: Implement a Quality Management System (QMS)
A robust Quality Management System (QMS) is the backbone of compliance. For most devices (above Class I), a QMS compliant with ISO 13485:2016 is mandatory. This system ensures that devices consistently meet customer and regulatory requirements. Your QMS should cover key areas like design controls, production, risk management (ISO 14971), corrective and preventive actions (CAPA), and supplier management. A well-implemented QMS is a critical tool for maintaining quality and streamlining compliance.
Step 3: Compile Technical Documentation and Conduct Evaluations
You must create a comprehensive Technical File demonstrating your device's conformity to the MDR or IVDR. This is the most substantial part of the process.
The Technical Documentation must include:
- Device description, specifications, and intended purpose.
- Labeling and Instructions for Use (IFU).
- Design and manufacturing information.
- A checklist demonstrating compliance with General Safety and Performance Requirements (GSPRs).
- Benefit-risk analysis and risk management file.
- Verification and validation data (e.g., bench, biocompatibility, electrical safety tests).
- Clinical Evaluation Report (CER) for medical devices, or a Performance Evaluation Report (PER) for IVDs, to verify safety and performance.
- A Post-Market Surveillance (PMS) plan.
You must also assign a Unique Device Identifier (UDI) for traceability. For a deeper dive, you can refer to the Details on EU product rules&from=EN).
Step 4: Engage a Notified Body (If Required)
For all devices except the lowest-risk Class I devices and Class A IVDs, you must engage a Notified Body. This is an independent organization designated by an EU country to assess product conformity.
Their role includes:
- Auditing your QMS against ISO 13485 and MDR/IVDR requirements.
- Reviewing your Technical Documentation to verify compliance.
- Issuing a CE certificate upon successful assessment.
You can Search for notified bodies in the NANDO database to find one designated for your device type.
Step 5: Issue a Declaration of Conformity (DoC) and Affix the Mark
After a successful conformity assessment, the manufacturer must draw up and sign an EU Declaration of Conformity (DoC). This is a legal declaration that the product meets all applicable EU legislation.
With the DoC complete, you can affix the CE mark to your device, its packaging, and documentation. The mark must be visible, legible, and indelible. If a Notified Body was involved, its four-digit identification number must be placed next to the CE mark. This signals that your device is now a Certified Medical Equipment ready for the European market.
CE Marking vs. FDA Approval: A Global Comparison
While CE marking and US FDA approval both aim to ensure device safety and effectiveness, they are distinct regulatory frameworks. Understanding these differences is crucial for manufacturers with global ambitions.
| Feature | EU CE Marking (MDR/IVDR) | US FDA Approval/Clearance |
|---|---|---|
| Governing Body | European Commission, National Authorities, and third-party Notified Bodies. | Food and Drug Administration (FDA), a single federal agency. |
| Regulatory Basis | Regulations (MDR 2017/745, IVDR 2017/746). | Federal Food, Drug, and Cosmetic Act (e.g., 21 CFR Part 820). |
| Core Philosophy | Presumption of Conformity: Manufacturer declares conformity, audited by a Notified Body for higher-risk devices. Focus on lifecycle and post-market surveillance. | Premarket Gatekeeping: FDA directly reviews and grants marketing authorization (510(k) clearance, PMA approval) before market entry. |
| Classification | Risk-based: Class I, IIa, IIb, III (MDs); Class A, B, C, D (IVDs). Rules-based system. | Risk-based: Class I, II, III. Based on intended use. |
| Conformity Process | Self-declaration for lowest-risk devices. Notified Body assessment of QMS and Technical File for higher-risk classes. | 510(k) Clearance for substantial equivalence. PMA Approval for novel, high-risk devices. De Novo pathway for novel, lower-risk devices. |
| QMS Standard | ISO 13485, integrated with MDR/IVDR requirements. | 21 CFR Part 820 (Quality System Regulation - QSR), which aligns with but is distinct from ISO 13485. |
| Clinical Evidence | Strong emphasis on device-specific clinical data, Clinical Evaluation Reports (CERs), and Post-Market Clinical Follow-up (PMCF). | Required for PMA and some 510(k) devices. Extensive clinical trials are often needed for novel, high-risk devices. |
| Post-Market | Robust Post-Market Surveillance (PMS), vigilance reporting, Periodic Safety Update Reports (PSURs), and the EUDAMED database. | Post-market surveillance, adverse event reporting, and recalls. FDA actively monitors devices on the market. |
| Validity | CE certificates are valid for up to 5 years, requiring annual surveillance audits. | FDA authorizations do not expire but require ongoing compliance. Significant changes may need new submissions. |
Key Differences in Regulatory Philosophy
The EU operates on a "presumption of conformity," where the manufacturer declares compliance, which is verified for higher-risk devices by a Notified Body. The focus is on a lifecycle approach with strong post-market surveillance. In contrast, the US FDA uses a "premarket gatekeeping" model, requiring manufacturers to get explicit marketing authorization from the agency before selling a device.
Impact on Global Market Strategy
These differences mean a global strategy requires separate regulatory submissions. A CE certified medical device does not automatically qualify for the US market, and vice-versa. Strategic planning must account for:
- Separate Submissions: Preparing unique applications for each market.
- Different Data Requirements: Clinical data may need to be supplemented or re-analyzed for each region.
- Timeline Variations: Approval timelines can differ significantly, impacting launch schedules.
- QMS Harmonization: A QMS must meet both ISO 13485 and specific FDA QSR requirements.
Navigating both systems requires regulatory expertise and careful planning from the start of product development.
Maintaining Certification and Post-Market Responsibilities
Obtaining CE marking is an ongoing commitment, not a one-time event. Maintaining certification and fulfilling post-market responsibilities are paramount once a CE certified medical device is on the market.
A Notified Body's CE certificate is typically valid for up to five years, contingent on continuous compliance. Manufacturers must pass annual surveillance audits that verify the QMS is effective and the device still meets all requirements. Any significant changes to the device or its manufacturing process must be reported to the Notified Body and may require re-assessment.
Failure to maintain compliance can lead to severe consequences, including market access prohibition, product seizure, fines, certificate withdrawal, and significant reputational damage.
Post-Market Surveillance for a CE Certified Medical Device
A cornerstone of the MDR and IVDR is the robust Post-Market Surveillance (PMS) system. This proactive process involves collecting and analyzing real-world data on a device's safety and performance to identify potential issues and take corrective action.
Key elements of PMS include:
- PMS Plan: A plan detailing the methods for proactive and systematic data collection, proportionate to the device's risk class.
- Post-Market Clinical/Performance Follow-up (PMCF/PMPF): A continuous process of collecting clinical data from a device's use to confirm its long-term safety and performance.
- Vigilance Reporting: An obligation to report serious incidents and field safety corrective actions (FSCAs) to the relevant national authorities.
- Periodic Safety Update Report (PSUR): For higher-risk devices, a report summarizing PMS data and analysis must be submitted regularly.
- EUDAMED Database: The European Database for Medical Devices (EUDAMED) is used to register devices and economic operators and report vigilance data, increasing transparency.
Effective PMS ensures a CE certified medical device is continuously monitored, protecting patients and allowing for timely interventions.
Frequently Asked Questions about CE Certification
Here, we address some of the most common questions about the journey to CE certification.
How long does it take to get a medical device CE certified?
The timeline varies significantly based on device classification, complexity, and Notified Body availability.
- Class I (self-certified): Approximately 3 to 6 months.
- Class IIa and IIb: Typically 9 to 18 months, requiring Notified Body involvement.
- Class III and Class D (IVD): Can take 18 to 24 months or longer due to stringent reviews and potential clinical investigations.
Key factors include the quality of your technical documentation, QMS maturity, and Notified Body responsiveness.
What are the costs associated with CE marking?
There is no fee for the CE mark itself. Costs arise from the conformity assessment process needed to prove compliance. These can vary widely and include:
- QMS Implementation: Costs for developing and maintaining an ISO 13485 compliant system.
- Technical Documentation: Resources for compiling the technical file, including testing and clinical evaluations.
- Notified Body Fees: Substantial fees for audits, technical file review, and annual surveillance.
- Testing Costs: Expenses for biocompatibility, electrical safety, and other required tests.
- Clinical Investigation Costs: A major expense if clinical trials are necessary.
- Other Fees: Costs for an EU Authorized Representative (for non-EU manufacturers) and regulatory consultants.
These costs are an investment in market access and patient safety.
Who is responsible for CE marking a device?
The manufacturer has the primary responsibility for ensuring compliance and affixing the CE mark. They must maintain the technical documentation and the EU Declaration of Conformity.
Other economic operators also have key roles:
- EU Authorized Representative: Required for non-EU manufacturers, this entity acts as the liaison with EU authorities.
- Importers: Must verify the device is CE marked and that the manufacturer has met its obligations before placing it on the EU market.
- Distributors: Must verify that the device bears the CE marking and meets labeling requirements before making it available.
While the manufacturer leads, all parties in the supply chain are crucial for maintaining the compliance of a CE certified medical device.
Conclusion: Your Next Steps in European Compliance
The path to becoming a CE certified medical device is complex, requiring a robust QMS and a deep understanding of the MDR or IVDR. While a significant investment, it open ups access to the vast European market and assures providers and patients of your product's safety and performance.
CE marking is a continuous journey of compliance, from initial classification to ongoing post-market surveillance. This commitment ensures legal market access and builds trust in the highly regulated medical device sector.
For procurement managers, understanding CE certification is key to making informed sourcing decisions. A CE certified medical device has met some of the world's highest safety standards.
At MedIX, we understand the importance of compliant medical equipment. Our global B2B marketplace simplifies procurement with AI-matching and rigorous compliance checks, connecting you with trusted suppliers of CE Marked Devices. We ensure transparency and reliability, so you can confidently Explore a wide range of CE marked devices and focus on patient care.
Expert voices
Insights from leaders transforming medical equipment procurement






More from our blog
Discover the latest trends in medical technology and procurement
Stay ahead of medical technology
Get the latest insights, research, and market updates delivered straight to your inbox
Ready to transform your procurement
Discover how MedIX can streamline your medical equipment sourcing process



